
The endocannabinoid system (ECS) has a lot to do with homeostatic processes within the brain. It controls mood regulation, pain perception, learning, and memory [1]. Much of the research that has been done to understand the role of the ECS in patients with drug dependencies has been with the use of cannabis.
ECS Receptors and Ligands
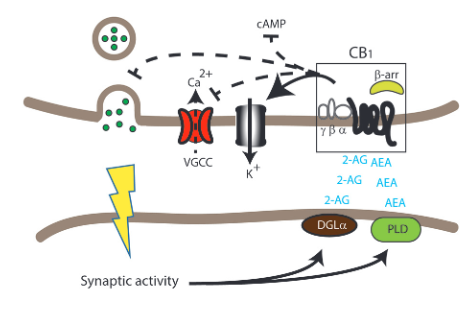
There are two main types of receptors of the endocannabinoid system, CB1 and CB2. The CB1 receptors are much more abundant, especially in presynaptic and axonal areas. These receptors are known for binding the active ingredient of Cannabis, delta 9-THC, and is responsible for the effects cannabis has on the central nervous system. CB1 receptors can also bind 2-arachidonylglycerol (2-AG) and arachidonylethanolamine (AEA) which are the two main endocannabinoids. Once these receptors are activated the levels of cAMP drop due to the inhibition of adenylyl cyclase. This inhibition stems from not having an agonist present.
The CB2 receptor is far less abundant, being found mostly in cells and tissues of the immune system. This receptor’s expression is usually involved in inflammation but can also play a role in synaptic plasticity and drug abuse. In a mouse model a CB2 receptor agonist led to inhibition of dopaminergic firing and less cocaine self-administration. The functions of the CB2 receptors have a role in Alzheimer’s disease. They have very selective localization and modulate microglia, which are highly associated with Alzheimer’s disease.
The ligands that bind the CB1 and CB2 receptors are known as endocannabinoids (eCBs). These molecules are produced when there is too high of a Ca2+ concentration within the cell. Endocannabinoids mediate feedback inhibition and modulate synaptic plasticity. The production of eCBs lead to a decrease of neurotransmitter release at either excitatory or inhibitory synapses. AEA and 2-AG act as receptor orthosteric agonists. Other allosteric eCBs are being identified and may have some therapeutic benefits by regulating the ECS.
One hypothesis explaining the process of selecting which signaling cascade is activated or inhibited has to do with phosphorylation by GPCR kinases that create targets for beta-arrestins. These targets are called bar-codes. Beta-arrestin signaling from the CB1 receptors can control the activation of different signaling cascades including the ERK pathways, JNK pathways, and the CREB cycle pathways.
Effects of Cannabis
As mentioned earlier, the active ingredient in cannabis, delta 9-THC, is what binds to the CB1 receptors. When humans are exposed to delta 9-THC they experience feelings of relaxation, auditory or visual illusions, pseudo hallucinations, and dissociation. In mice, exposure to delta 9-THC results in antinociception, hypothermia, hypoactivity, and catalepsy [1]. These psychoactive symptoms are due to the fact that delta 9-THC acts as a partial agonist of the CB1 receptor.
There is still an immense amount of research needed to be done on the affects of cannabis and THC on the human brain. Currently, there is only one university that is allowed to partake in cannabis use for research purposes. Knowing more about the harmful and therapeutic effects of cannabis use will be very beneficial for its possible use for medicinal purposes and even recreational purposes.
CNS Diseases
Disorders localized in the central nervous system, specifically Alzheimer’s disease, Huntington’s disease, and multiple sclerosis, can benefit from some therapeutic qualities of cannabis or delta-9 THC. An oromucosal spray known as Sativex can relieve motor dysfunction and pain symptoms in people with multiple sclerosis and help with neuroprotection for people with Huntington’s disease. Synthetic cannabinoids can reduce inflammation and pain.
References
- Kendall, D. A., & Yudowski, G. A. (2017). Cannabinoid Receptors in the Central Nervous System: Their Signaling and Roles in Disease. Frontiers in Cellular Neuroscience, 10. https://doi.org/10.3389/fncel.2016.00294