Glioblastoma (GBM) is a cancerous brain tumor. GBM is a grade IV cancer with the prediction of a 14-month survival period upon diagnosis for most cases and a 5-year survival prediction in less than 5% of cases [1]. Glioblastoma can be divided into two main categories, primary and secondary GBM. Secondary tumors develop from pre-existing lower grade tumors whereas primary tumors develop on their own. An example of secondary glioblastoma can be from an overgrowth of astrocytes. Diffuse astrocytoma (grade II) can develop into anaplastic astrocytoma (grade III) which then goes on to develop into glioblastoma (grade IV) over the course of several years [1].
Types of GBM
There are four main types of glioblastomas. Classical GBM is associated with the overexpression of the growth factor receptor gene EGFR and the tumor suppressor protein TP53. Mesenchymal GBM is associated with alterations in specific components of the PI3K and MAPK pathway, NF1, a negative regulator of the MAPK pathway, and PTEN, a negative regulator of the PI3K pathway. Both classical and mesenchymal GBM best respond to aggressive treatment forms. Proneural GBM is most commonly found in younger patients and has to do with an amplification of a platelet growth factor gene, PDGFRA, found on chromosome 4q12. This form of GBM is less responsive to aggressive treatment. Lastly, neural GBM does not have any obvious mechanistic patterns but is associated with the expression of neuronal genes. This form has the shortest survival rate.
Invasiveness of GBM
GBM is a very invasive cancer, causing treatment to be difficult. Complete surgical removal of the tumor is nearly impossible. Matrix-metalloproteinases are thought to allow GBM cells to break down the extracellular matrix and therefore contribute to its invasiveness because of the upregulation of these proteins found in GBM patients. This upregulation is due to dysfunction of several signaling pathways. The dysregulation of signaling pathways is thought to be a promoting factor of this cancer.
Signaling and Crosstalk
The signaling pathways thought to be involved in glioblastoma are the PI3K, MAPK, and cAMP pathways. In the MAPK and PI3K pathways are hyperactivated, whereas the cAMP pathway is hypoactive. Both the MAPK and PI3K pathways have regulation roles for each other along the signaling cascade. When Ras, a molecule that has a role in activating Raf in the MAPK pathway can also activate PI3K. Targeting Ras could potentially be a very beneficial mode of treatment for cancer patients due to the link it has between two different pathways involved in GBM, but the development of this treatment may be difficult due to the molecule’s small size. As mentioned above, the cAMP pathway is hypoactive in GBM patients. Inhibition of PDEs can inhibit tumor growth and promote apoptosis of cancer cells which can also be a potential treatment option after more research is conducted. The CREB transcription factor is a crossing point between all three pathways and could also provide insight into targeting common factors.
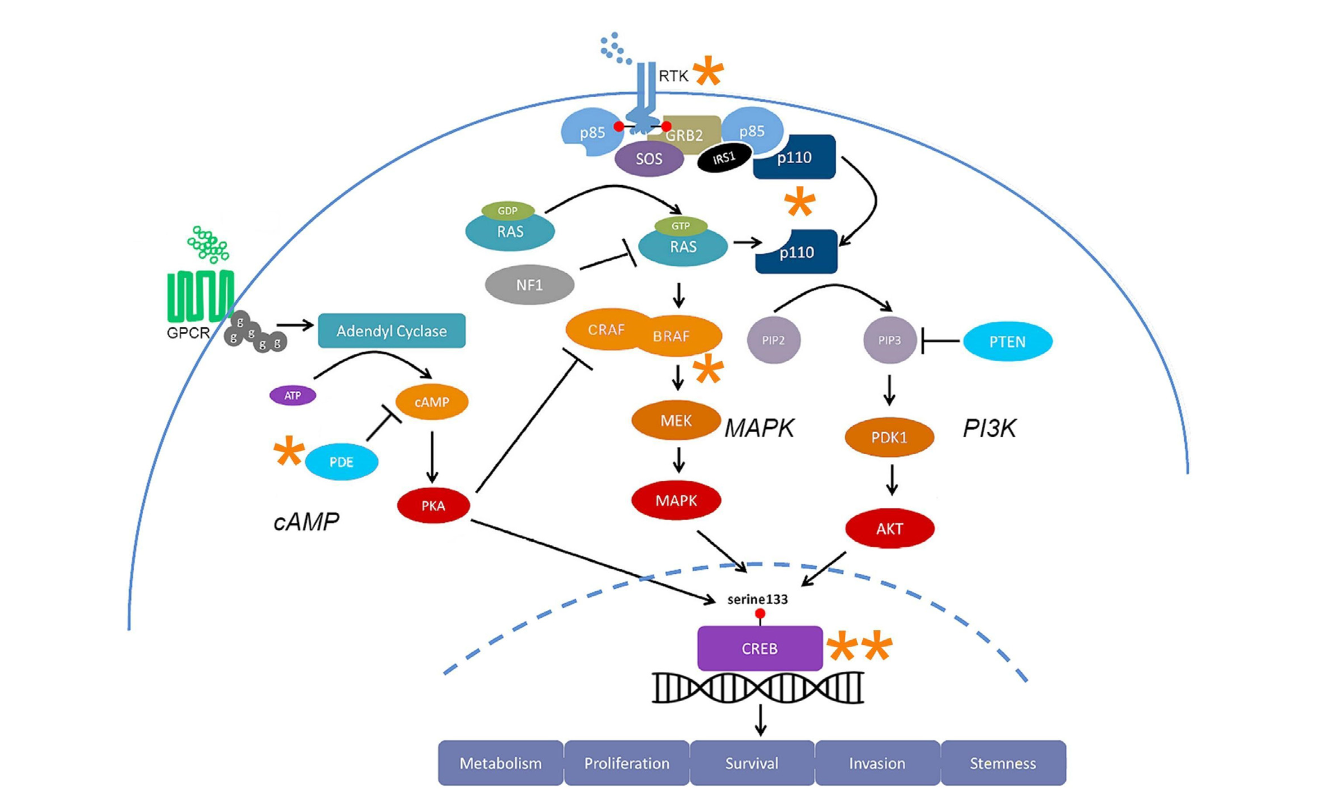
There are specific signaling molecules that could be potential options to target for the treatment of glioblastoma. Because there are multiple pathways involved in the carcinogenesis of glioblastoma, it may be ineffective to target just one pathway, so finding regions of overlap may be more efficient to target when considering different treatment options than the typical chemo and radiation. This can bring up concerns about ensuring specific drug combinations are safe for patients.
Glioblastoma is a very serious cancer that doesn’t give affected patients many options for survival and treatment. Learning more about the intermolecular and intramolecular signaling could assist in providing better treatment options for patients.