Until recently the brain was thought to be a insulin-insensitive organ. It has been discovered that there are various actions of insulin in the brain. Insulin acts on the hypothalamus to control brain metabolism and body energy balance. Insulin signaling also plays an important role in all aspects of memory. It is required for synaptic plasticity and learning.
Amyloid plaques are a key trait of Alzheimer’s and for a long time was thought to be the reason for memory loss. After examining brains post mortem there is no correlation between the amyloid plaques and memory loss. The cognitive decline was found to be connected to synapse damage.
Alzheimer’s and diabetes have very similar pathophysiological and clinical traits. Brains with Alzheimer’s disease have defective insulin signaling which leads to synaptic dysfunction and memory issues.
There is currently research being done to discover if the mechanisms that underly impaired peripheral insulin signaling in type 2 diabetes and alzheimer’s are related.
So what does this mean? This means that there may be a direct link between the underlying mechanisms of type 2 diabetes and Alzheimer’s. The current mechanism of Alzheimer’s is pretty unclear. It cannot be diagnosed until after death and because of that it is even more difficult to study.
Now we see that having a healthy lifestyle may not only save you from type 2 diabetes but also Alzheimer’s disease.

That might not be enough for some people, fortunately diabetes are a well researched and treated field. It is difficult to treat Alzheimer’s, because we don’t understand the mechanism. Finding this connection could lead to some treatment choices after the damage has begun. Using anti diabetic treatments for patients with memory loss could be on the horizon.
Diabetes and Alzheimer's: a relationship few have thought about.
The topic for this week in Neurochemistry, the class of wonderful authors featured on Cobbers on the Brain, was a topic that hits home for millions of people around the world: Alzheimer’s disease. Having never done extensive research on the topic, I was very curious to know how and why this devastating disease developed, but just like almost all neurological diseases, the possibilities seemed to be endless. The article we discussed explained the possible connection between diabetes and Alzheimer’s development. More specifically, it looked at insulin resistance that characterizes type 2 diabetes and its role in the disease.
For both Alzheimer’s disease and diabetes, altered cell metabolism, inflammation, and insulin resistance are key pathological features in both diseases so this led researchers to explore a possible relationship between the two. It is also well known that insulin plays a role in the regulation of Beta-amyloid, the protein that builds up to form plaques in patients with Alzheimer’s. Insulin receptors have also been found to be key component in memory formation and have been found to be compromised in early stages of Alzheimer’s disease. All of these relationships direct a bulk of current research to the thought that insulin resistance, like seen in diabetes, could be directly related to the development of Alzheimer’s later in life.
What do these findings mean for Americans today?
For our society with increasing rates of obesity and diabetes, these findings should be alarming. The relationship between insulin resistance and Alzheimer’s could mean that as America continues to become a population with high rates of diabetes, more and more people could be at greater risk for developing Alzheimer’s disease.

(#artstracts)
On a positive note, this research has opened doors to the possibility that insulin resistance treatment in the brain could eventually be a treatment for Alzheimer’s disease. If it could be determined how to overcome the insulin resistance in Alzheimer’s patient brains, then the development of amyloid plaques could be reduced and memory function increased.
As research continues on this topic, I think it’s important to recognize that our lifestyle choices today always have the potential to greatly impact our future. If the thought of obesity and diabetes isn’t scary enough, always try and remember to think about that precious brain of yours!
The Rise of a New Era?
Will the older generations begin to look different with the rise in type II diabetes? Recently more and more studies have been shown to prove that having type II diabetes greatly increases the risk of developing Alzheimer’s disease.
The way our society has been eating and the lack of exercise as a whole has contributed to an exponential growth in the amount of individuals who now have diabetes. With this being known, will the baby boomer generation cause an increase in the number of Alzheimer’s disease patients?
In Alzheimer’s disease brains, large plaques of amyloid-beta oligomers (ABO’s) are found and is thought to be one of the main components in cognitive impairment. The initiation of Alzheimer’s disease is still unknown but is thought to be caused by a various collection of impairments, such as endoplasmic reticulum (ER) stress, malfunctioning insulin signaling, and inflammation.
Another large factor in the progression of Alzheimer’s disease is the loss of insulin receptors in the brain. Insulin is produced in the pancreas and travels through the blood. Once near the brain, it crosses the blood brain barrier. When, for example, someone has insulin resistance, insulin is not properly being used and the brain is then affected.
Receptors are continually being recycled into the cell through clathrin-coated pits and replaced for maintaining the cell membrane. But in Alzheimer’s disease, insulin receptors are not being replenished back into the membrane, inhibiting the amount of insulin that needs to bind and then enter the cell. Insulin begins to accumulate in the synaptic cleft, and this is where research is still unclear but is thought to cause a cascade of events that ultimately leads to neuronal death and memory loss.
 With all that we know about the relationship between insulin resistance in type II diabetes and Alzheimer’s disease, our society should begin thinking about the potential rise in Alzheimer’s disease patients throughout the United States.
With all that we know about the relationship between insulin resistance in type II diabetes and Alzheimer’s disease, our society should begin thinking about the potential rise in Alzheimer’s disease patients throughout the United States.
Questions arise like, how we will take care of these individuals and whether or not we should be putting more money into researching a cure so that it can be prevented as much as possible. With no concrete future direction it is up to us to inform others and begin taking better care of our bodies.
Diabetes and Alzheimer’s: Eating Healthy Results in More Than Just Weight Loss
In Neurochemistry class, we talked about Alzheimer’s Disease, which is one of the most detrimental disorders that someone can experience. We talked about how there are a lot of different theories for the etiology of the disorder (i.e. genetics) and other associated factors.
We learned about Amyloid-Beta plaques and oligomers, which are basically just strands of misfolded/dysfunctional proteins that disrupt neuron signaling, eventually leading to cell death and, moreover, cognitive decline.

One of the most interesting things that I learned, however, was the association between Alzheimer’s Disorder and Type II Diabetes. Current research is explaining how LDL, or “bad cholesterol,” is linked with Alzheimer’s in that it can lead to the increase in AB build-up in the brain which, as mentioned before, is also concomitant with AD.
In a review done in 2013, they mentioned multiple studies that have linked Type II Diabetes to Alzhiemer’s, and how that association has increased substantially in the last few years. It is estimated that it is because of the Westernized lifestyle of high-caloric food intake with a lack of exercise.
The study also mentioned how they have found that both AD and Type II Diabetes have insulin resistance, which results in an increased amount of blood sugar and, therefore, a higher potential for AB plaques and cell death.
Insulin plays a very important role in metabolism and protein synthesis. Basically, we need this hormone to form memories and process our food. Without it, we would experience cognitive impairments such as memory loss.
Current research is showing promise for insulin treatment and the slowing of Alzheimer’s symptomology, although more research is needed to confirm this hypothesis.

The take-home message that I absorbed through our last Neurochemistry class was that eating healthy and exercising does more than just help you lose weight. There is a substantial amount of supporting evidence that eating right and working out can lead to less “bad cholesterol” build-up, and decrease your risk for Type II Diabetes and, therefore, potentially decreasing your risk for Alzheimer’s disease.
Again, more research is needed to fully understand how to fully prevent this order, but I now know to eat more apples and walk to class more than I currently do.
Good News for Meredith Grey? An Investigation into the Mechanisms of Alzheimer’s Disease
For all of the Grey’s Anatomy fans out there, in season 9 we discover that Meredith does in fact carry the gene for Alzheimer’s—inherited from her mother. The discovery is dire.
And while we were reassured by McDreamy’s consoling of Meredith—in that genes don’t determine our fates—the fact of the matter is Meredith is at risk for this detrimental disease.
It is also likely that an increasing number of our population is at risk. In this week’s research, our class found links to insulin intolerance and the development of Alzheimer’s Disease (AD).
With type II diabetes diagnoses on the rise, this increased insulin intolerance could lead to a rise in Alzheimer’s Disease diagnoses in the coming years.
This makes the study of AD imperative, and the search for treatments, as well as preventative measures all the more a priority.
The good news? Our understanding of the chemical underpinnings of the disease are increasing, and the research we have delved into seems to suggest we might be on the brink of finding the true root of the problem.
The Importance of Insulin
Recent research has shown the importance of insulin in the brain. Insulin naturally crosses the blood brain barrier and regulates many important functions in neurons.
When a person experiences insulin resistance, this could result in less of the vital insulin signaling functions in the brain, leading to memory impairment and AD development.
Decreased insulin signaling also leads to increased tau phosphorylation. Tau is an enzyme in the brain that’s normal functioning is important for microtubule structure in axons of neurons. When phosphorylated, tau’s actions are inhibited—thus creating issues with axon signaling.
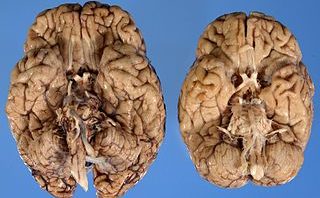
Phosphorylated tau protein combines and aggregates into plaques or neurofibrillary tangles (NFTs). These NFTs have been major signs of AD for many years and were originally thought to be the cause of AD.
Thus, the new found link between tau and insulin signaling marks the continued uncovering of the AD pathology.
What came first, the chicken or the egg?
With all this good news, we could almost leap for joy for Meredith! If she avoids Type II Diabetes she will be in the clear!
However, although scientists are close to unveiling the root of the cause, insulin resistance has not been proven to be the sole culprit of AD development—alas, we cannot celebrate for Meredith quite yet.
While insulin resistance does not help your chances for avoiding AD, there is also a second piece to the puzzle—this being AβOs. These oxidative stress agents are also thought to cause AD.
The AβO molecule induces stress on neurons of the brain, resulting in less insulin signaling, which perpetuates stress, which leads to kinase stress response, which increases more AβO formation, which begins the cycle again.
The question becomes: Which came first, the AβOs or the insulin resistance?

(#artstracts)
It could be that either/or could lead to AD, and that because one influences the other, a bad combination of the two could lead to the same result.
For example, say Meredith Grey avoids the junk food and does not develop insulin resistance via type II diabetes. She is still predisposed genetically, and she has a stressful job. The stressful job of being a surgeon could lead to an increase in AβO production, which could lead to insulin resistance in the brain, which would cause stress, and start up the AD development cycle.
Or, for example, say Meredith does hit the junk food hard. She develops type II diabetes, becoming insulin resistant. This insulin resistance is experienced in her brain as well as the rest of her body, and this begins putting stress on her neurons. The neurons respond with stress kinases, which lead to AβO production, which begins the cycle of AD development.
Either way, AD becomes the issue, and this is where the research lies.
If this is truly good news for Meredith or not is surely debatable. However, the research is showing more promising answers to our questions of the root of AD development.
The hope is that if we can get to the root, we can find a treatment for this detrimental disease.
If we can find a treatment, then surely that will be good news for Meredith. But until then, we’ll just hope she relaxes, exercises, and avoids the junk food.
Alzheimer's disease and insulin resistance
Research into the molecular nature of Alzheimer’s Disease has been a major focus the past 20 years, with (AD) claiming about 90,000 Americans annually. With genetic factors attributing to AD very minimal, the mechanisms of sporadic, late-onset AD is still not fully clarified. Intense research efforts have sought to identify this complex neurological disorder the past few years.
Recent research has unveiled the connection between Alzheimer’s disease and Insulin signaling, indicating a brain-specific form of diabetes. Several clinical studies have now established factors including hyperglycemia and hyperinsulinemia has correlated positively with the development of Alzheimer’s development in humans. Overall, Alzheimer’s brains have exhibited malfunctioning insulin pathways, and a largely decreased responsiveness to insulin.
After years of belief that insulin has no belonging in the brain, numerous studies have revealed insulin actions are key for neuronal survival and brain function. It is now established insulin regulates brain metabolism and energy balance in the hypothalamus, and is important for memory formation, association, and retrieval in the forebrain and hippocampus. Other functions include having neuroprotective properties and having a large role in cognitive functioning.
neuronal survival and brain function. It is now established insulin regulates brain metabolism and energy balance in the hypothalamus, and is important for memory formation, association, and retrieval in the forebrain and hippocampus. Other functions include having neuroprotective properties and having a large role in cognitive functioning.
For the last 15 years, Alzheimer’s disease has been distinguished by a few specific characteristics: senile plaques and neurological tangles. Senile plagues are composed of large clumps of amyloid fibrils, specifically amyloid-β (Aβ) peptides. However despite a proven toxicity of these large aggregates, many post-mortem observations showed that the large amyloid plagues did not correlate well with AD symptoms. Instead, a loss in plasticity or neuronal signaling was discovered to be associated with oligomers of Aβ peptides called AβO’s. These toxins are now considered responsible for synapse loss and triggering Alzheimer’s Disease symptoms.
In conditions such as type 2 diabetes, sustained metabolic stress and inflammatory signaling lead to decreased insulin signaling and a decreased biological response to insulin. This is called insulin resistance, and this significantly impairs your body to properly store energy in your blood, so characteristically glucose levels soar through the roof with diabetes patients. Interestingly, this same process occurs in the brain with insulin signaling in the occurrence of Alzheimer’s disease. Several recent studies have linked neuropathic mechanisms triggered by AβOs to mechanisms involved in insulin resistance in diabetes.
Overall, if AD mechanisms are from the same causation as type 2 diabetes, these conditions can be prevented from occurring through maintaining a healthy lifestyle. The most important factor in preventing diabetes, and eventually Alzheimer’s disease, is limiting your amount of body fat. Developing excess weight near your abdomen has been closely linked to insulin resistance. This is because this deep fat isn’t near your skin, and is more likely to cause insulin resistance compared to fat developed on your hips and thighs.
Eating a diet of high-fiber, slow-release carbohydrates is an important step in a healthy diet. Carbohydrates have a big impact on your blood sugar levels, so having a smart approach to what types of carbohydrates you eat is important. Complex carbohydrates include foods like brown ride, whole-wheat pasta, whole-wheat foods, high-fiber cereals, peas and leafy greens.
Overall, several studies have shown a link of insulin resistance to the increasingly common Alzheimer’s disease. Insulin resistance has shown to have little genetic connections, so a healthy lifestyle and diet are key controllable aspects that will ultimately decrease your chances of Alzheimer’s disease.
Parkinson's Disease: Finding the Balance
Merriam webster’s dictionary defines Parkinson’s disease as “a chronic progressive neurological disease chiefly of later life that is linked to decreased dopamine production in the substantia nigra and is marked especially by tremor of resting muscles, rigidity, slowness of movement, impaired balance, and a shuffling gait.”
A nice balance between dopamine and acetylcholine is key to motor control. Dopamine also plays an important role in many other bodily functions and neurotransmissions. For Parkinson’s disease the biggest issue is the imbalance between dopamine and acetylcholine, because of the decreased dopamine production.
Researchers so far have had a difficult time pinpointing what is causing the neuronal damage leading to Parkinson’s and other related diseases. Some environmental factors for Parkinson’s disease include increased age, being a male, head injury, occupation, area of residence, , pesticide exposure, exposure to metals, genetic predisposition, solvents and PCBs.
For a lot of these factors we can’t really do a whole lot until it is too late and the damage has already begun. These factors also don’t necessarily lead to Parkinson’s, but do increase your chances.
There is no way of getting around the fact that an increase in age will increase your chances of Parkinson’s and being a male will also increase your chances. Acquiring a head injury could lead to any number of related issues, but those are typically out of a person’s control. The occupation you choose could have some side effects that come with it depending on what you are exposed to. Jobs where you may be exposed to toxic chemicals and metals could increase your chances of developing disease.
Where you live could be a factor in your Parkinson’s chances. It could be environmental factors of the specific area that you live in, but it could also be related to the gene pool in your area. Maybe the fields around you are spraying pesticides or the place where you get your produce uses chemicals that could lead to an increased risk. Say you take away pesticides, then we have more of a risk acquiring an insect transported disease.
The Parkinson’s Disease Foundation also listed some potential protective factors. Caffeine, anti-inflammatory drugs, exercise, and vitamin D have been found to have positive effects on the brain. Some controversial protective factors included uric acid, taking drugs that lower your cholesterol, and nicotine.
Males with high amounts of uric acid tend to form kidney stones, but they also have an lower risk of developing Parkinson’s. If you have high cholesterol and take drugs to lower your cholesterol then you will have a lower chance of getting Parkinson’s, but your health wasn’t good to begin with because you had high cholesterol. If you naturally have low cholesterol your chances of developing Parkinson’s disease is higher. There has been a correlation between smoking and neuronal damage. Researches believe that nicotine may block the destruction of the neurons that leads to Parkinson’s.

These simple everyday factors are a lot like the proteins and enzymes in our body that we talk about in class. In one place they are doing a really great thing, but in another place they are destroying us. Why would our body be producing things that our toxic to us? With almost everything we do there are potential benefits and drawbacks. Our bodies weren’t meant to live forever and longer we live the more we are beginning to understand and fight that.
The Imbalance of Parkinson's Disease
Commonly seen in people over the age of forty, approximately 60,000 United States citizens are diagnosed with Parkinson’s disease every year. The magnitude of its affect along with its serious implications on the duration and the conditions of life give imperative reasons to understand this disease. While I do not believe medical practices should be catered to extending life, I do believe that modern medicine should seek to suppress its cognitive and motor affects. The only way to develop an affective treatment plan is to continue extensive research on the pathophysiology of this disease.

Characteristically, Parkinson’s disease causes the death of dopaminergic neurons in the portion of the brain known as the substantia nigra. Because of these neuronal deaths, patients suffer from dyskinesia which is the inability to control voluntary muscle contraction. Typically, patients display tremors, rocking back and forth, or fidgety movements. The real question, however, is what causes these neurons to die and why are these specific neurons affected?
It has been found through many neurodegenerative studies that the build up the protein, alpha-synuclein, is responsible for this dopamingeric neuronal death. Typically, this protein is found in the brain of healthy individuals, but at much lower concentrations. This is because the proteosome complex (proteins that breakdown other proteins) of healthy individuals use the enzyme Heme-oxygenase-1 to breakdown high levels of alpha-synuclein. In Parkinson’s disease patients, however, their brain has become resistant to this enzyme so there is no way to breakdown this protein leading to an aggregation of alpha-synuclein and eventually dopamingeric neuronal death. The use of heme-oxygenase-1 is a specific enzyme that is used in these cells to breakdown alpha-synuclein which leads to this neuronal specific cell death.

In order to suppress the impairment of Parkinson’s disease, it is essential to reestablish homeostasis in the part of the brain by degrading alpha-synuclein. Because of this, future research should focus on determining a reason why the brain becomes resistant to Heme-oxygenase-1 and develop a drug that could degrade this protein. If these goals were achieved, many friends, families, and patients would not have to go through the hardships caused by this disease.
As a student seeking a career in the medical field I think that focusing on this type of research would have many benefits to society as whole, not just those affected by Parkinson’s. This is because the knowledge gained in a study like this will take another step toward understanding the neurochemistry of the brain, hopefully leading to answers as to why these types of mutations arise in certain individuals. With this information we could better understand the impact of genetics and environment on neurodegenerative disease and help physicians detect and prescribe medications for these individuals. While I do not think we should seek to chase immortality, I do believe that as a society we should value the conditions of life and the complexity of our minds.
Parkinson's Disease: Another Result of Brain Injury?
I’m willing to bet that you have heard of the boxing legend Muhammad Ali. If you haven’t, I would definitely recommend watching some of the highlights of his illustrious career in the boxing ring. The guy was amazing. However, the guy also took some very heavy blows to the head throughout his time in the ring. That’s not surprising, though – boxing is an extremely brutal sport that comes with high risk of  injury. But research into the neurological effects of traumatic brain injury suggest that this “injury” may be more than just a temporary painful experience. The unfortunate reality of Muhammad Ali’s life today is that he suffers from Parkinson’s Disease, or PD, and the connection between his diagnosis and history of boxing raises many questions.
injury. But research into the neurological effects of traumatic brain injury suggest that this “injury” may be more than just a temporary painful experience. The unfortunate reality of Muhammad Ali’s life today is that he suffers from Parkinson’s Disease, or PD, and the connection between his diagnosis and history of boxing raises many questions.
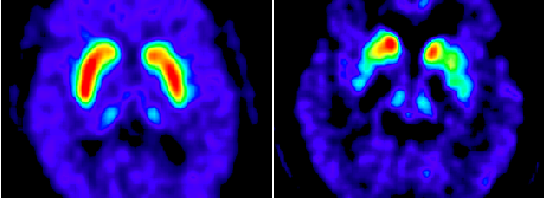
What exactly is PD? It is widely known that PD is a progressive disorder of the nervous system that affects movement – a symptom that stems from the loss of dopaminergic neurons in the substantia nigra, a region in the midbrain. The role of abnormal protein kinase function, which leads to the elevated phosphorylation of the PD-defining pathological protein alpha-synuclein, has been implicated in having an effect on the loss of neurons. This depletion of neurons, shown in the picture below, leads to a decreased supply of dopamine in the brain, which is responsible for many functions such as movement control. Typically, PD patients exhibit this loss of control through slowed and jerky movements. This video shows the effects of PD on Muhammad Ali himself, in comparison to before he developed the disease.
But why did he develop PD? Could there be a connection to the years and years of taking punches to the head? Researchers at UCLA have done extensive work on traumatic brain injury (TBI) and its connection with the development of Parkinson’s Disease (PD). They have found that having experienced a TBI doesn’t necessarily cause PD, but dramatically increases the risk of developing the disease. One of the researchers leading the study explained that, “With a moderate traumatic brain injury, the loss of neurons was too small in number to cause Parkinson’s disease, but it is enough to increase the risk of PD. By decreasing the number of dopaminergic neurons, any further insult to the brain will be attacking a smaller number of neurons; as a result, the threshold for symptoms would be reached faster.”
the risk of developing the disease. One of the researchers leading the study explained that, “With a moderate traumatic brain injury, the loss of neurons was too small in number to cause Parkinson’s disease, but it is enough to increase the risk of PD. By decreasing the number of dopaminergic neurons, any further insult to the brain will be attacking a smaller number of neurons; as a result, the threshold for symptoms would be reached faster.”
In the long-term study, found here, the rats’ brains showed a 30 percent loss of dopaminergic neurons 26 weeks after the injury. This finding suggests that traumatic brain injury alone is sufficient to induce a progressive degeneration of dopaminergic neurons in the long term. Additionally, this research supports the finding that with a first moderate brain injury, the susceptibility to another increases drastically.
Isn’t this a scary thought? Not only does an injury to the brain lead to immediate effects such as concussion development, but it can also lead to the loss of neurons responsible for coordinating muscle movement in the body. And with the buzz surrounding the concussion policy of the National Football League as well as the seemingly endless cases of chronic traumatic encephalopathy (CTE) former NFL players have developed, it seems that the impacts of brain injury are even more severe than previously imagined.
Is Deep Brain Stimulation the New Face for Parkinson's?
In the United States, more than 200,000 people are affected each year from Parkinson’s disease. As much of the general public knows, this disease is a debilitating neurodegenerative disease that often is diagnosed after the age of 40. That being said, what many people don’t know is that the symptoms that they associate with Parkinson’s disease may not be from the actual disease itself, but rather the medications individuals are taking. Possibly the most recognizable characteristic of advanced Parkinson’s is dyskinesia, or involuntary motor movement that can been seen as body tremors, rocking back and forth, or just the inability to sit still. Surprisingly, this hallmark of the disease often is the result of high doses of drugs derived from the compound called L-DOPA.
What We Know about Parkinson’s
Truly, the symptoms in the disease’s original state are slowness of voluntary movements, a shuffling gait, abnormal stiffness, and poor balance. These are the result of the death of dopaminergic neurons in an area of the brain called the substantia nigra. In Parkinson’s disease, the leading theory is that this cell death is caused by the build-up of an activated protein called Alpha-synuclein. Little is known about why this activated protein builds up in dopaminergic neurons, but extensive research is being done on kinase dysfunction that could lead to the buildup and subsequent cell death. Knowing that activated Alpha-synuclein is likely the cause for the dopaminergic cell death in Parkinson’s disease, there is also clinical research focused on targeting the Alpha-synuclein, including vaccines and compounds that can break down the protein.
In past and many current treatments for Parkinson’s disease, the goal has been to supplement dopamine for the brain to try and compensate for the death of these neurons. Dopamine drugs, including L-DOPA, serve this purpose well. This drug has the ability to flood the brain with dopamine, but unfortunately, patients taking high doses of this drug have to live with the side effects such as the inability to sit still.
Where Deep Brain Stimulation Fits in
This is where deep brain stimulation (DBS) may be the key for some Parkinson’s patients. The goal of this procedure is to regulate the “on/off” characteristics of the disease and help reduce dyskinesia. In DBS, a thin electrode is implanted into the brain, targeting motor circuits that are not functioning properly. Small electrical pulses from a device similar to a cardiac pacemaker are then used to stimulate a small brain region and block the signals that cause some Parkinson’s symptoms and symptoms from L-DOPA drugs. DBS may not be the answer for everyone, but studies have shown that is works best for patients who respond well to dopamine drugs but just suffer from their side effects. Just as with any other medication or procedure, this treatment should be approached with realistic expectations and understanding of the risks associated.
For me, as a student infatuated with research and its clinical applications, this is extremely exciting; but I think this also should be exciting for everyone. Deep brain stimulation may be a way for people suffering from Parkinson’s to experience the relief from drugs such as L-DOPA, but also live a life not hindered by its nasty side effects such as dyskinesia. With this brain treatment, before research is developed enough to effectively target the Alpha-synuclein and find a cure, there is a possibility that people with Parkinson’s may have the ability to be just another face in the crowd.