When we talk about tumors, they can grow and exist differently. There are canceorus (malignant) tumors and non-cancerous (benign) tumors. A tumor is a solid mass of tissue that forms when abnormal cells group together. They can affect different parts of the body including the bone, skin, tissue, and organs. Factors that increase the risk of developing a tumor include gene mutations, smoking, family history of certain types of cancer and smoking. [1]
Glioblastomas (GBM) are brain tumors that affect the normal intracellular and intercellular signaling for the advantage of the tumor cells but to the disadvantage of the whole organism. Some of the subtypes of glioblastomas include classical GBM, mesenchymal GBM, proneural GBM, and neural GBM.
GBM can also occur as a primary tumor or a secondary tumor developing from pre-existing lower grade tumor glioma tumors. Primary GBM develop very quickly, without evidence of preexisting symptoms while secondary GBM develop from a lower grade tumor. Some of the common symptoms of glioblastomas include perisistent headaches, double or blurred vision, vomiting, new onset of seizures, changes in mood and personality. [2]
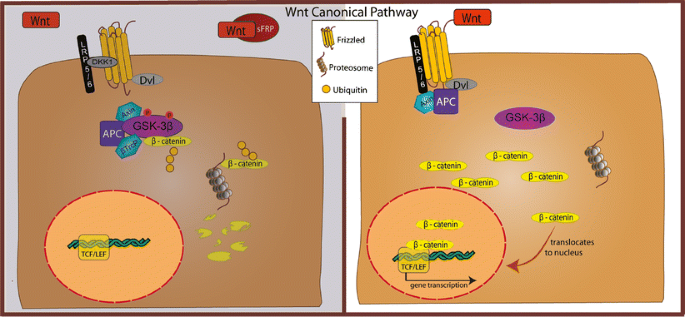
To delve more into the neuroscience of cellular processes involved in GBM, the Mitogen- activated protein kinase (MAPK) signaling and pathways are interrupted in GBM. This can affect functions such as cell survival. The MAPK pathway contains three activated protein kinases that are key components of a series of vital signal transduction pathways and regulte regulate proceses such as cell proliferation, cell differentiation, and cell death. Below is a diagram showing the MAPK pathways. [3]
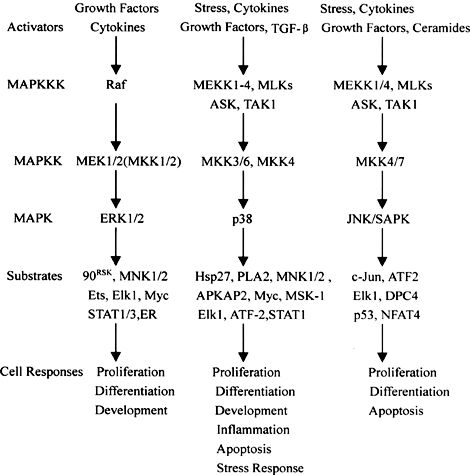
Figure 1. Diagram showing the major MAPK pathways (cascades) in mammalian cells [4]
Think of the MAPK pathway like a chain reaction in your body’s cells that gets started when certain growth factors, like epidermal growth factor (EGF), bind to their special receptors on the cell surface. These receptors are like switches that turn on the pathway. When the growth factor binds to the receptor, it causes the receptor to team up with other proteins inside the cell, like GRB2 and SOS. These proteins then pass signals along to another protein called Ras, which acts like a messenger. Ras gets activated and starts a series of events that ultimately lead to the activation of MAPK, a protein that helps control cell growth and division.
In cancer, this pathway can go haywire. Sometimes, certain genes like the one for the epidermal growth factor receptor (EGFR) get too active, causing the MAPK pathway to go into overdrive. This can make cells grow and divide uncontrollably, leading to tumor growth and making the cancer more aggressive.
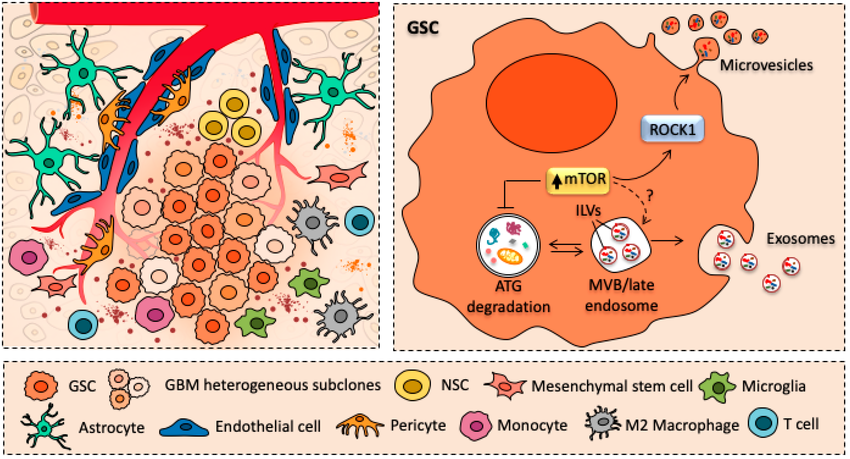
Figure 2. Cartoon schematizing the crostalk between glioblastoma cancer stem cells (GSCs) and major cellular components of glioblastoma tumor environment.
References.
[1] Professional, C. C. medical. (n.d.-a). Tumor: What is it, types, symptoms, treatment & prevention. Cleveland Clinic. https://my.clevelandclinic.org/health/diseases/21881-tumor
[2] Jigisha, T., Pier, P., & Vikram, P. (n.d.). Glioblastoma multiforme. AANS. https://www.aans.org/en/Patients/Neurosurgical-Conditions-and-Treatments/Glioblastoma-Multiforme