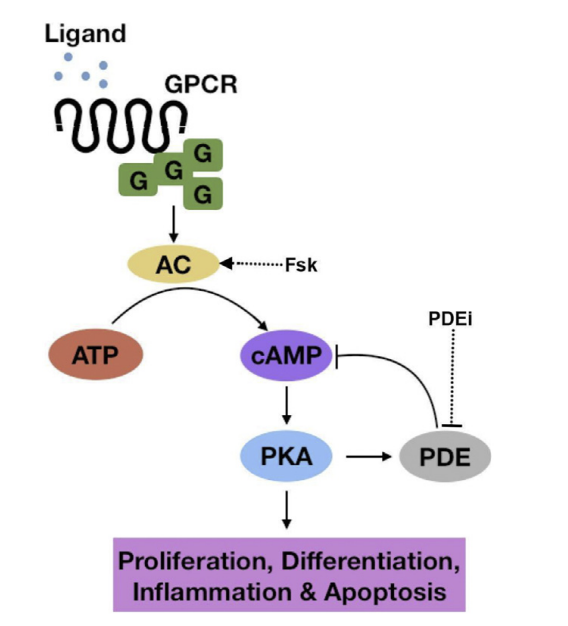
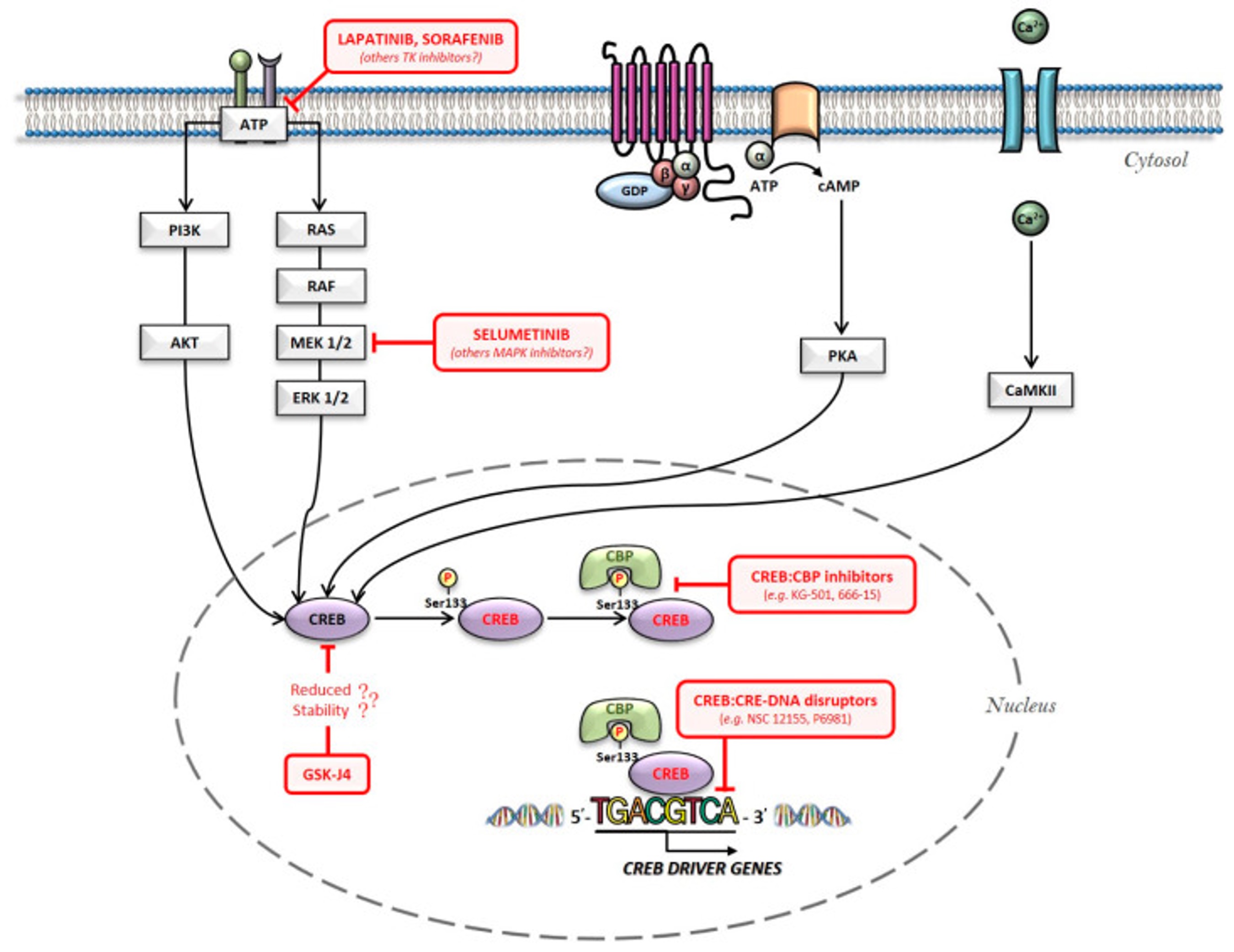
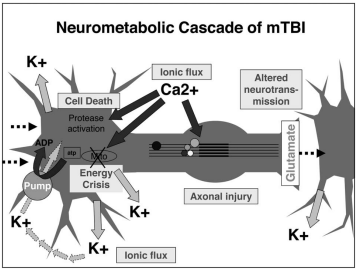
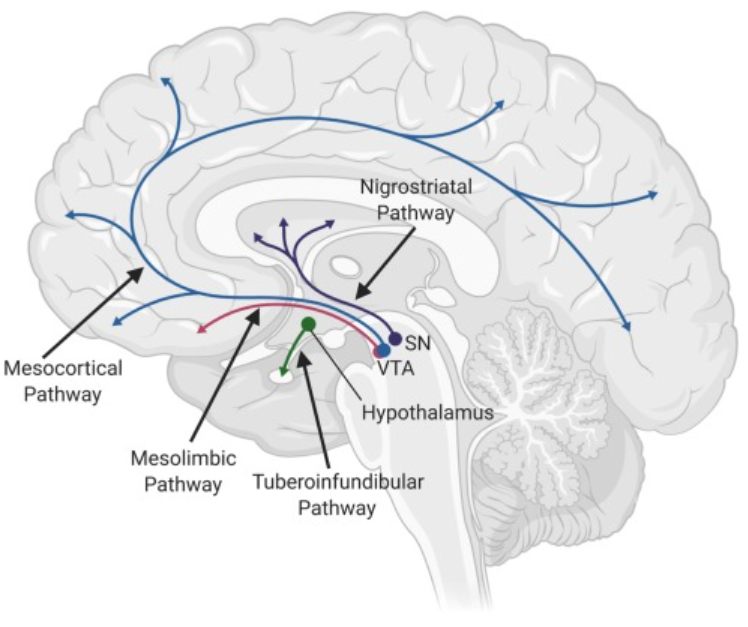
Addiction is a treatable, chronic medical disease involving complex interactions among brain circuits, genetics, the environment, and an individual’s life experiences. [1] Addiction has been a part of cultures for as long as people have been using addictive substances. The most common ones are caffeine, nicotine, and alcohol. [2] Addiction affects the brain and affects how a person’s life circumstance influences the use of addiction, it involves factors such as cultural, psychological, and genetics of a person. Many individuals who become addicted, struggle in knowing when to stop. When a person takes a drug, it affects their “reward circuit “This induces feelings of euphoria and triggers a surge in the neurotransmitter dopamine when a reward system is functioning properly, individuals engage in essential behaviors such as eating nutritious food and spending time with loved ones, when dopamine levels increase it strengthens the association between taking drugs and the feeling of joy they produce, consequently individuals are more likely to be involved in these behaviors despite the possibility of bad outcomes all due to dopamine making them feel good. When a person continuously takes drugs, their brain will start to adapt to the drug and adjust by making cells in the reward system less responsive to it, individuals won’t feel the same way after taking the drug multiple times this becomes known as tolerance. Some individuals during this time will increase drug intake, in an attempt to experience a “high” such as before, the changes in the brain can cause pleasurable activities to decrease such as eating and spending time with others, long long-term effects can affect learning, judgment, decision making and memory of an individual. [3] Drug abuse is increasing and it’s important to understand what abuse means and its relation to addiction. Abuse is when a drug or prescription is used in a way that is not intended, abuse can start in any way, and at first, individuals may not be aware of this, such example would be grounding up pills and snorting and/or injecting the pill to get high. The problem with doing this is that these prescription drugs “most often misused include opioid painkillers, anti-anxiety medicines, sedatives and stimulants.”[4] Consequences of abusing prescription drugs can lead to serious life-threatening risks, and ultimately death. Mu Receptors (mu-opioid) receptors are GPCRs, they regulate many functions in the brain, and they are mainly found in the central nervous system. Mu receptors are part of the body’s endogenous opioid system, which regulates pain, reward, and addictive behaviors. [5] When opioid molecules attach to mu receptors on brain cells in the LC (locus coeruleus), they reduce the release of neurotransmitter NA (norepinephrine), causing effects like drowsiness, slowed breathing, and low blood pressure—common signs of opioid intoxication. But with continued opioid use, the LC cells adapt by becoming more active. So, when opioids are around, this increased activity balances out their effects, and the person feels normal. However, when opioids aren’t present, the LC cells release too much NA, leading to symptoms like restlessness, anxiety, cramps, and diarrhea. [6] The FDA has approved medications to treat addiction, but that doesn’t eliminate it, but they’re also resources out there for individuals experiencing addiction, with advancements in technology, hopefully, scientists are medical professional can find more treatment options for this addiction crisis we are facing.
https://www.yalemedicine.org/news/how-an-addicted-brain-works
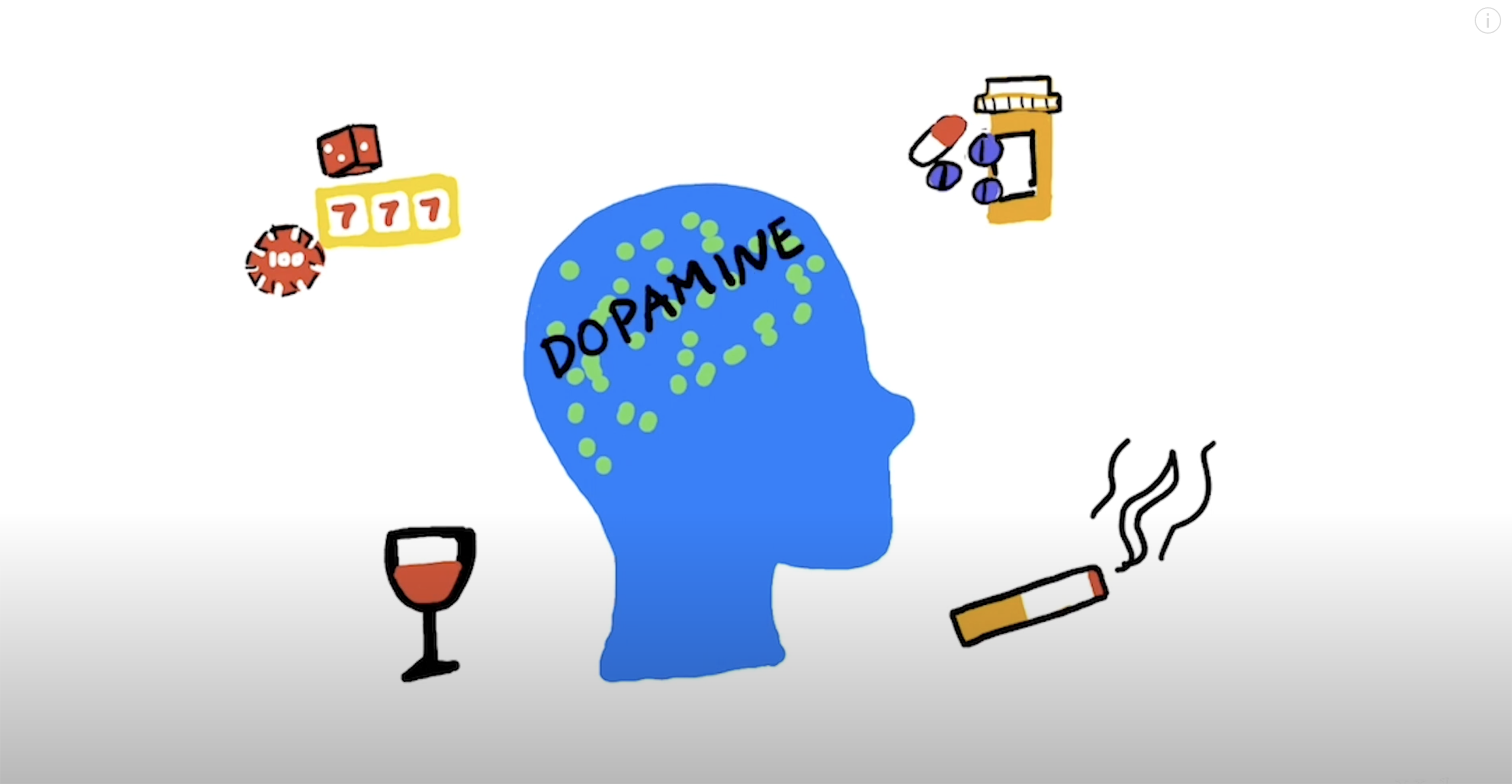
[1] “What Is the Definition of Addiction?” What Is the Definition of Addiction? , American Society of Addiction Medicine, 2024, www.asam.org/quality-care/definition-of-addiction.
[2] Crocq, M.-A. (2007). Historical and cultural aspects of man’s relationship with addictive drugs. Dialogues in Clinical Neuroscience, 9(4), 355–361.
[3] NIDA. “Understanding Drug Use and Addiction DrugFacts.” National Institute on Drug Abuse, 6 Jun. 2018, https://nida.nih.gov/publications/drugfacts/understanding-drug-use-addiction Accessed 20 Mar. 2024.
[4] “Prescription Drug Abuse.” Mayo Clinic, Mayo Foundation for Medical Education and Research, 25 Oct. 2022, www.mayoclinic.org/diseases-conditions/prescription-drug-abuse/symptoms-causes/syc-20376813.
[5] Volkow, Nora D et al. “The addicted human brain: insights from imaging studies.” The Journal of clinical investigation vol. 111,10 (2003): 1444-51. doi:10.1172/JCI18533
[6] Herman, Timothy F. “Mu Receptors.” Mu Receptors, U.S. National Library of Medicine, 30 July 2023, www.ncbi.nlm.nih.gov/books/NBK551554/#article-25311.s6.