Artstract made in Canva
Concussions are well understood by some, usually those who have had a concussion or been in concussion prone settings like sports, but not understood by many [1]. Athletes and their parents probably are aware of the symptoms and signs of concussions, but another aspect is how the concussion impacts the brain (no pun intended). Concussions directly result from an injury to the head which then affects the brain. However, is the brain bruised? Fractured? Sprained? It can be quite unclear how the brain is exactly hurt after a concussion.
In medical terms, a concussion is called a mild traumatic brain injury (TBI); however, don’t confuse their meaning with mild [2]. Mild in this case means non-life threatening. [2]. In their effort to increase concussion awareness, the CDC (center for disease control) has created this video to explain what a concussion is [2]. The head receives some type of trauma that causes the sudden movement of the brain back and forth inside the skull [2]. I mentioned sports earlier, because contact sports like football, soccer, hockey, etc. are the places that people and kids are most likely to get a concussion [1].
The next question is:
What happens to your brain after a concussion?
A neuroscience article by Doctors Giza and Hovda, provides insight into how the brain reacts to a concussion on the cellular level [1].
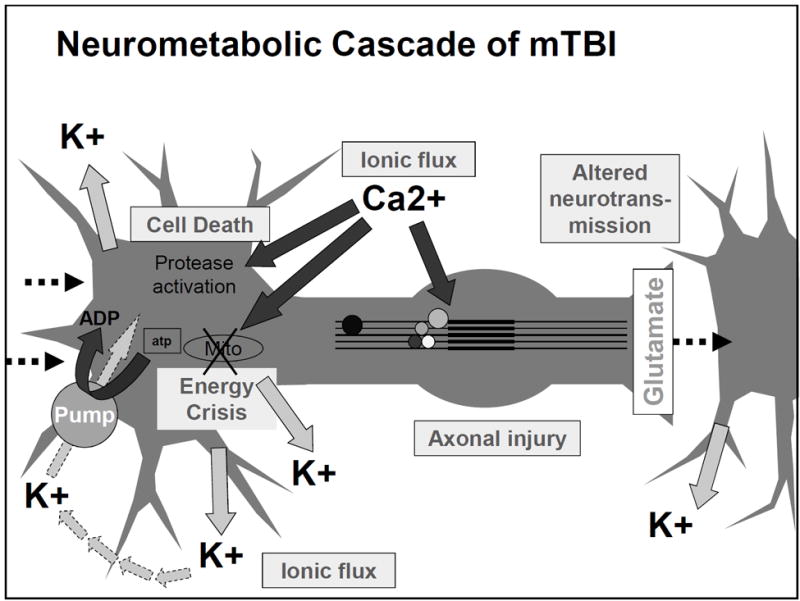
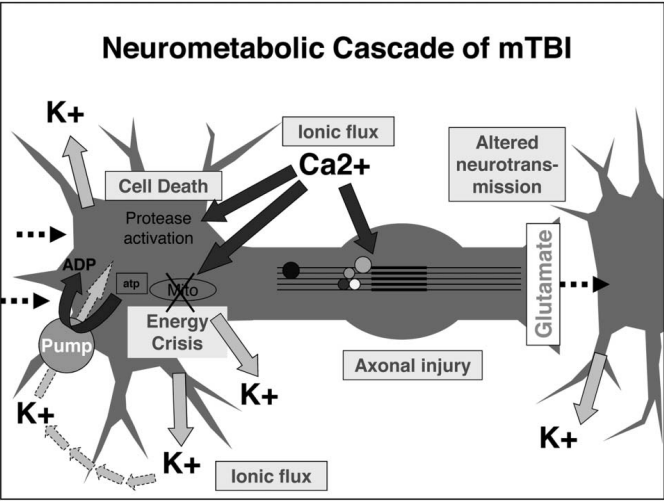
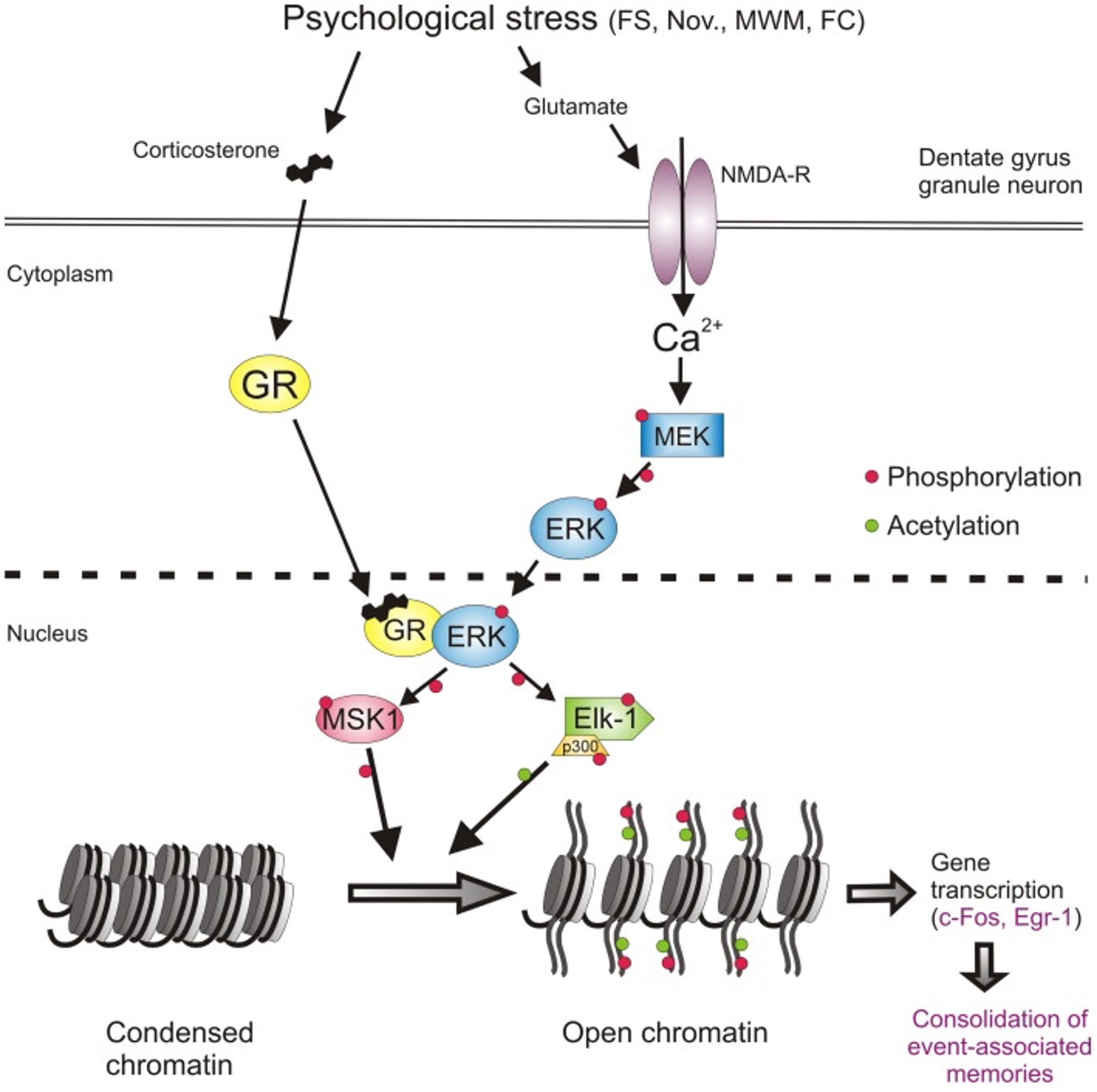
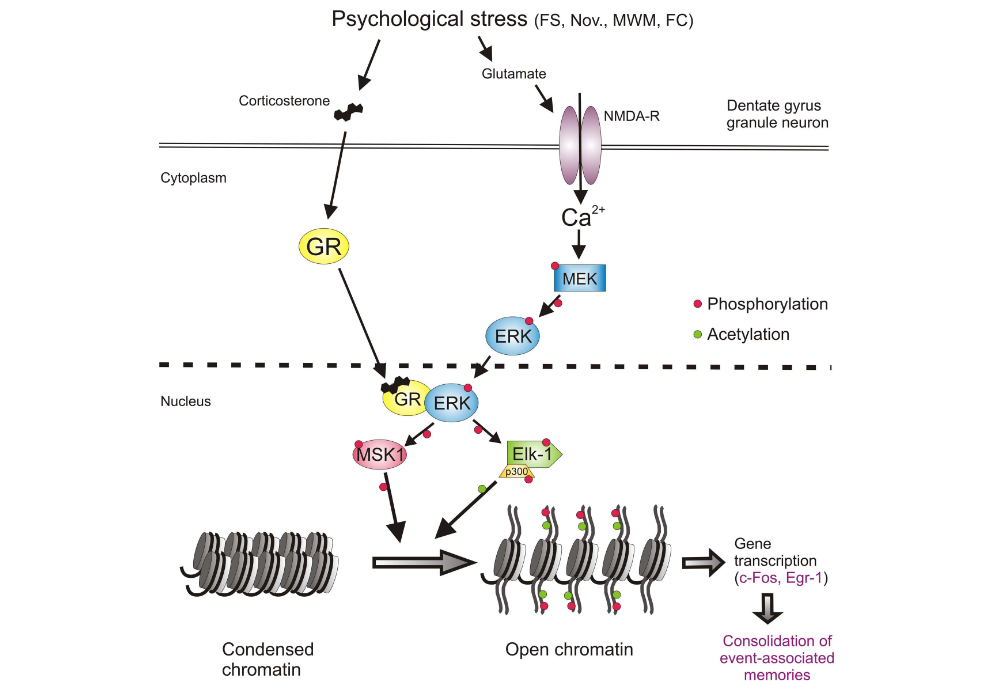
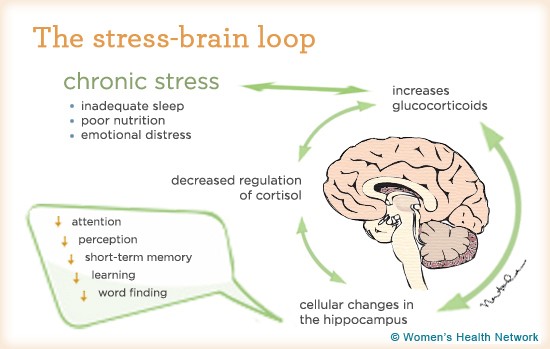
One of the first things after your brain rattles, is an ionic flux, which means all the ions flow rapidly into the brain cells. An ion is a small atom with a charge, and the most common one in the brain is the calcium ion also shown as Ca²+ [3]. Ions are key to making the brain work regularly, but like an overfilled balloon, the brain cells can stretch and burst if there are too many ions (air) in the cell (balloon). The medical term for this swelling is called cerebral edema [2].
 [4]
[4]
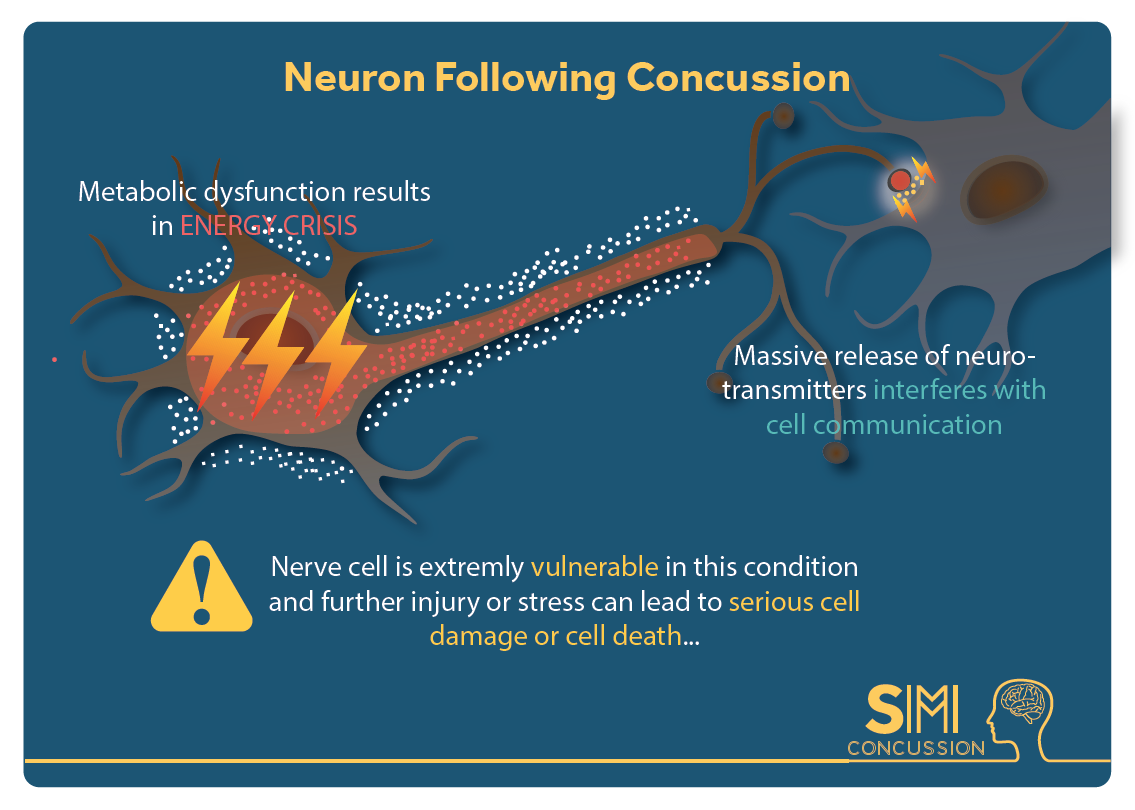
In addition to swelling, the ions that flow into the cell will flow into the mitochondria, “the powerhouse of the cell” [1]. This puts stress on the mitochondria. Since the mitochondria is the power source for the cell, if it’s not functioning properly due to stress, the whole cell cannot function properly; this can result in an energy crisis [1].
Your body tries to handle this energy crisis by going into hyperglycolysis. Hyper = more than normal, and glycolysis is how your body consumes glucose (sugar and carbohydrates) that you get from your diet. So your body is trying to use all of its energy to solve this mitochondria stress situation in the brain. This initial period of hyperglycolysis is followed by hypoglycolysis wish is essentially the opposite. Your body isn’t processing food into energy as much as it should. This lessened glucose consumption happens for 7-10 days in adults [1].
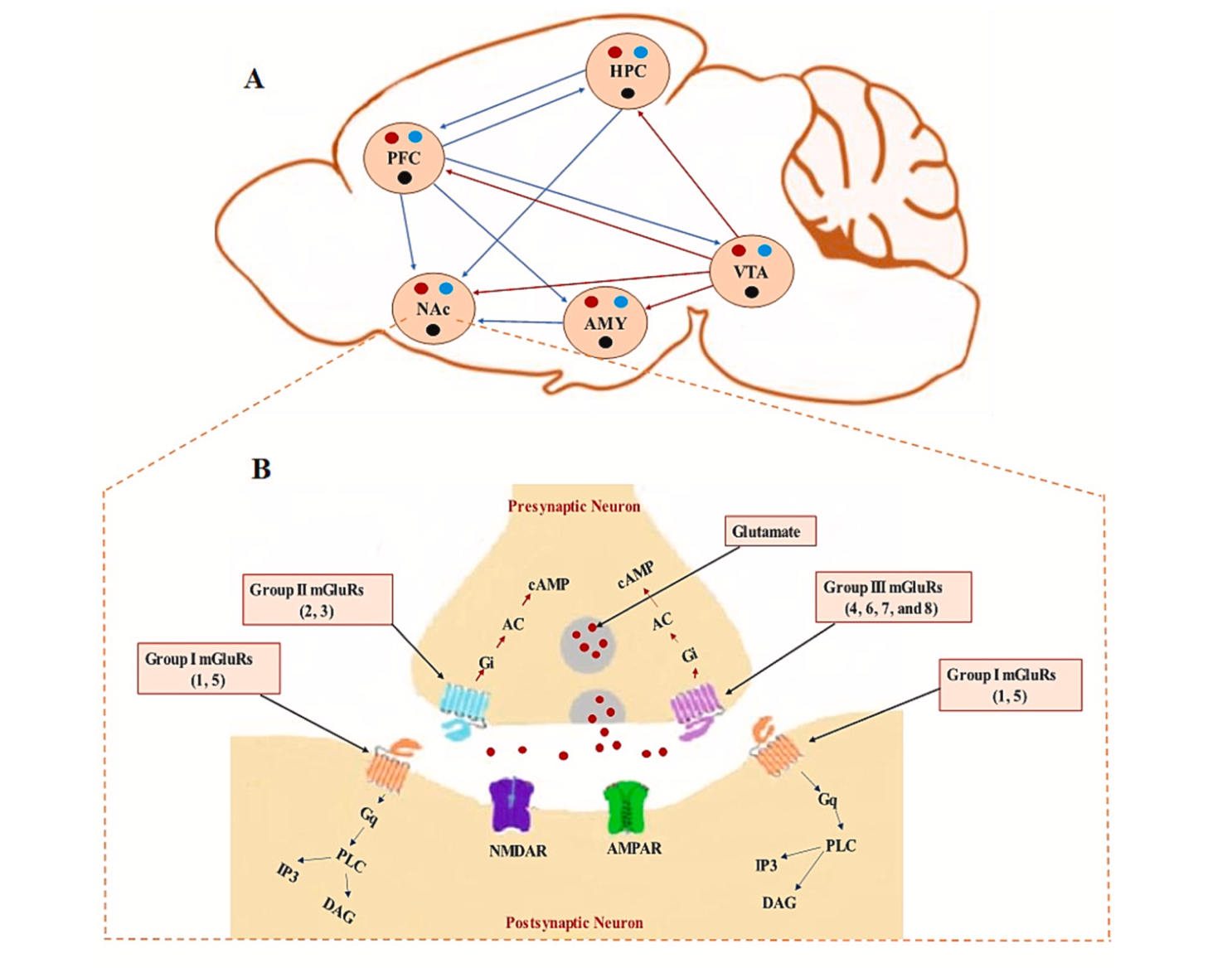
What I have pointed out about ionic flux and the energy crisis can be seen in this figure from the article by Giza and Hovda. I would like to emphasize the movement of Ca²+ into the cell and then towards that mitochondria labeled Mito in the figure.
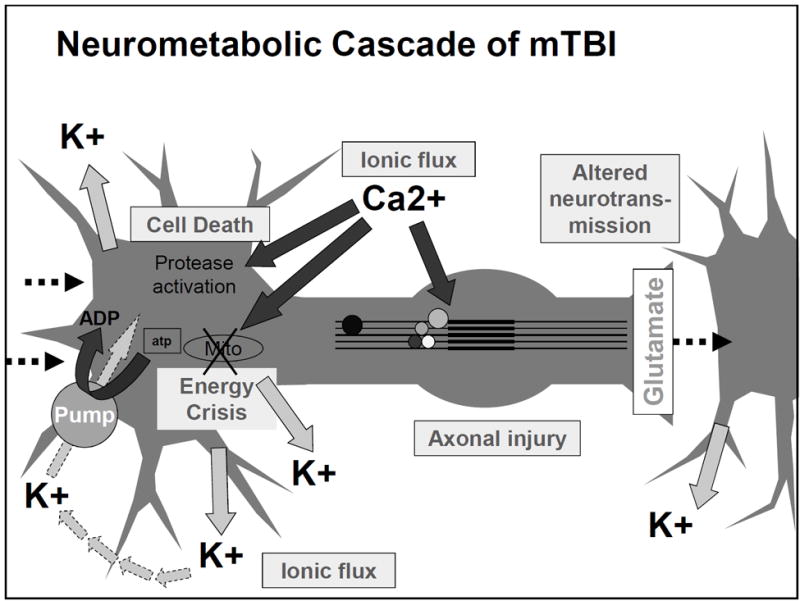 [1]
[1]
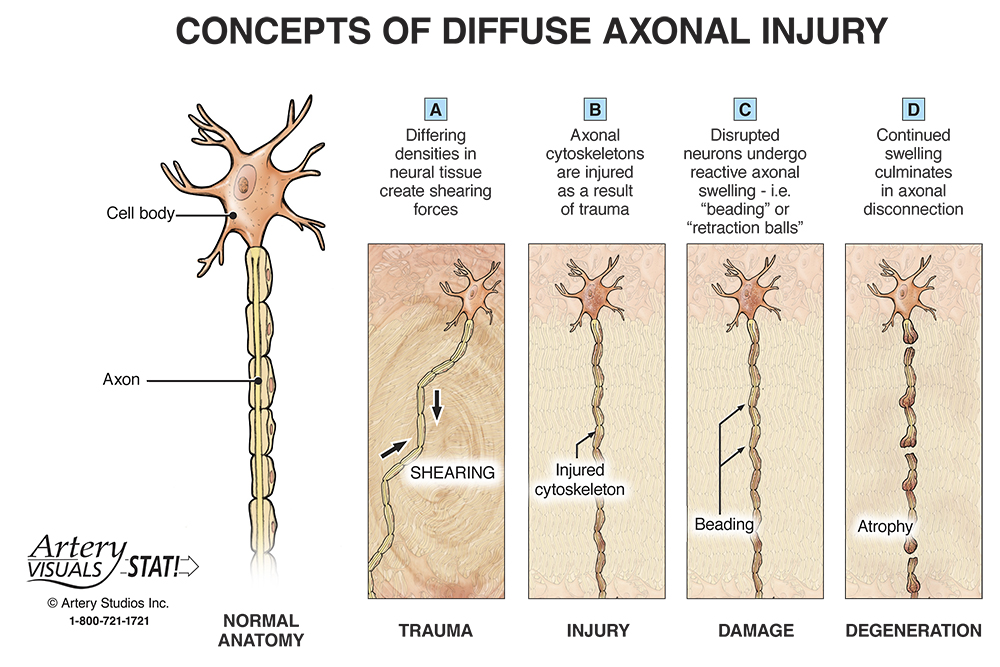
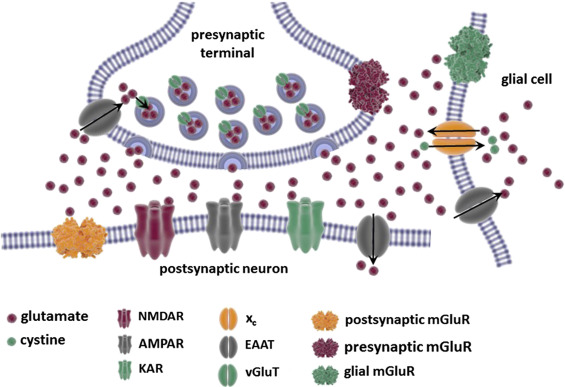
In addition to ions not moving correctly, and the body not consuming energy normally, there can be serious structural damage. The most common place for these injuries in brain cells is the axon. In the diagram below you can see that the axon is this long thin section of the brain cell. This thin structure makes it really sensitive to the jostling and rattling the brain experiences in a concussion. However, when an axon is damaged the brain can’t send signals properly; this is when you get the symptoms of confusion and memory loss in concussions. Luckily, axons can be repaired after damage, but it takes time like all construction and repairs [1].
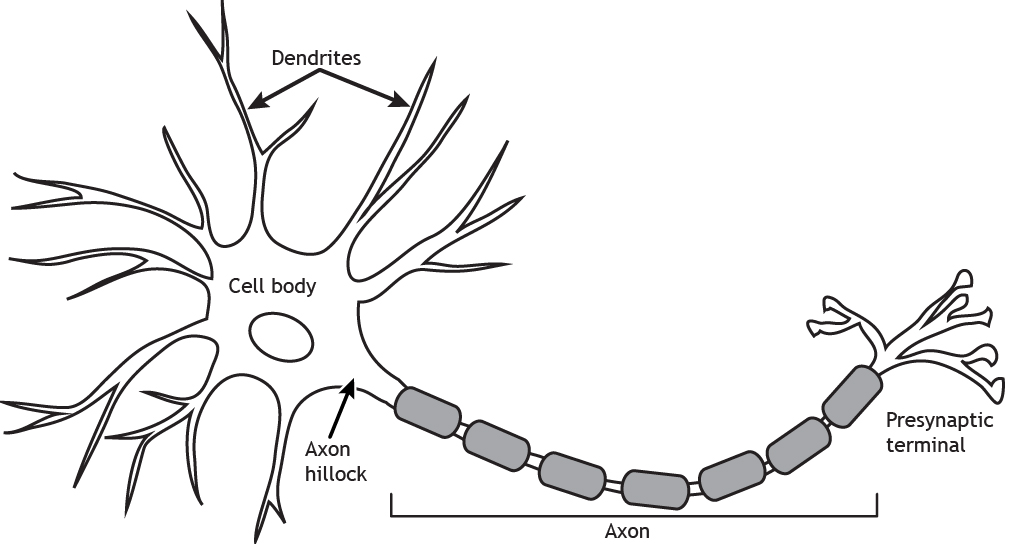 [5]
[5]
These are three of the major occurrences at the cellular level in the brain after a concussion. Your brain is doing these changes in ions, glucose consumption, and axon repair in order to try and heal. The brain is trying to get things back in working order, but, like in all injuries, healing takes time. I am hoping that this discussion of things on a small scale can help you to understand how a concussion works and why healing is so important after a concussion.