How is Traumatic Brain Injury Classified?
- Traumatic Brain Injury (TBI) occurs when there is a sudden injury to the head, such as a concussion, resulting in damage to the brain. This damage can range from producing mild and temporary consequences to severe and long-lasting effects that can potentially permanently reduce various cognitive functions.
- When a concussion occurs, there are a myriad of physiological consequences that ensue within the brain, leading to headaches, impaired cognition, and other common symptoms that are often associated with this form of injury. The cellular mechanisms and structures affected by the impact include the increased flux of various key ions, energy deprivation, cell death, and damage to neurons that results in a lack of neuronal communication throughout the brain. The duration of these impairments can range from acute to chronic, as numerous factors, including recovery time and repeated trauma, dictate the course of the injury.
The Hypothalamus and the Pituitary Gland
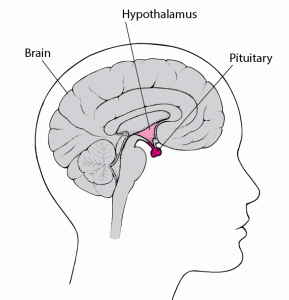
- The two main players involved within the relationship between TBI and the menstrual cycle are undeniably the brain regions of the hypothalamus and the pituitary gland. A vital role of the hypothalamus and pituitary gland is to control the functioning and regularity of the menstrual cycle. The hypothalamus triggers the pituitary gland to synthesize and release specific hormones that prompt the ovaries to make estrogen and progesterone. Estrogen and progesterone will then prepare the endometrium for pregnancy until fertilization occurs. If fertilization does not occur, estrogen and progesterone levels decrease, resulting in the shedding of the endometrium, otherwise known as menstruation.
- Since the pituitary gland and hypothalamus are located in the inferior aspect of the brain and unprotected by layers of brain tissue, these brain structures are at greater risk of damage from trauma from when an injury to the brain does indeed occur.
- Injury to this region of the brain often results in hypopituitarism, a phenomenon in which the pituitary gland is unable to properly synthesize and release the necessary hormones for adequate bodily functioning. In a study with 1,000 TBI patients, hypopituitarism occurred in 27.5% of the cases. This finding demonstrates the high probability of endocrine malfunctioning following TBIs as a result of damage to the pituitary gland and consequent hypopituitarism.
Now What is the Relationship Between TBI and the Menstrual Cycle?
- One of the most notable effects of endocrine disruption is the impact on the routine pattern of the menstrual cycle. Both irregularities in the menstrual cycle and amenorrhea (absence of menstruation) have been shown to be a primary consequence of damage to the pituitary gland from TBI. This damage results in the hypopituitarism state mentioned above, in which there is a lack of sufficient estrogen and progesterone that can sustain a normal, regular menstrual cycle.
- This finding is strengthened by the fact that a common consequence of pituitary gland tumors in women is the symptom of amenorrhea.
- Other key findings:
- Research has shown a correlation between the duration of amenorrhea and severity of brain injury, as more severe TBIs result in a longer period of amenorrhea and vice versa.
- Many women who have experienced one or more TBIs report a greater intensity of menstrual cramps during menstruation following the injury.
Looking Ahead
- As novel guidelines and options for treatment continue to advance within the realm of TBIs, the consequences of endocrine disturbances as a result of brain damage, including amenorrhea and menstrual cycle irregularities, should be further recognized and researched.






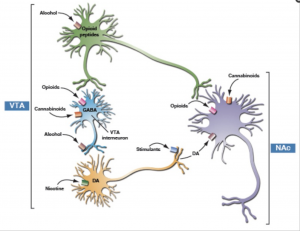
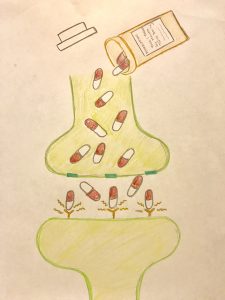
 disorder”. For instance, participants that have internet abuse behaviors had higher impulsivity, or in other words, a lower response inhibition. They had a more difficult time inhibiting a natural response to wanting to play a game or browse social media. These participants also had a heightened activation of brain regions that deal with reward response. The responses seen through MRI were positively correlated with self-reported urges to game. The correlation here to drug addicts would be that not only are the same brain regions activated, but these urges are similar to drug users experiencing cravings, which are neurological in nature but manifest to influence behaviors. Another way in which this addictive quality can be seen with chemical changes in the brain is through looking at the dopaminergic system. In drug addicts, the binding capacity for dopamine receptors is diminished, which means that drug users need more of that drug (or higher doses) to feel the same amount of high as they have before. This is also referred to as tolerance. This reduced dopamine capacity was also found in internet abuse participants. In participants that were identified as having gaming related addictions, their binding potential (or ability to feel good while playing their game) was diminished in ways that rivaled an injection of amphetamines, a highly addictive (and psychoactively responsive) drug.
disorder”. For instance, participants that have internet abuse behaviors had higher impulsivity, or in other words, a lower response inhibition. They had a more difficult time inhibiting a natural response to wanting to play a game or browse social media. These participants also had a heightened activation of brain regions that deal with reward response. The responses seen through MRI were positively correlated with self-reported urges to game. The correlation here to drug addicts would be that not only are the same brain regions activated, but these urges are similar to drug users experiencing cravings, which are neurological in nature but manifest to influence behaviors. Another way in which this addictive quality can be seen with chemical changes in the brain is through looking at the dopaminergic system. In drug addicts, the binding capacity for dopamine receptors is diminished, which means that drug users need more of that drug (or higher doses) to feel the same amount of high as they have before. This is also referred to as tolerance. This reduced dopamine capacity was also found in internet abuse participants. In participants that were identified as having gaming related addictions, their binding potential (or ability to feel good while playing their game) was diminished in ways that rivaled an injection of amphetamines, a highly addictive (and psychoactively responsive) drug. 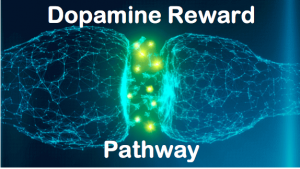
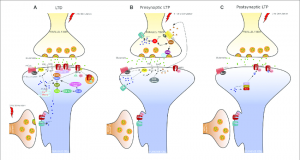

 cascade of events that lead to gene transcription caused by CREB or delta-FosB. Through this cascade of events leading to gene transcription, neurons can become altered whether that be in size or shape, or even in the amount of receptors that are found on the synapse. These changes are what lead to the effects of addiction: sensitization, compulsive drug-seeking, loss of control, reward, dependence, withdrawal, tolerance, and relapse.
cascade of events that lead to gene transcription caused by CREB or delta-FosB. Through this cascade of events leading to gene transcription, neurons can become altered whether that be in size or shape, or even in the amount of receptors that are found on the synapse. These changes are what lead to the effects of addiction: sensitization, compulsive drug-seeking, loss of control, reward, dependence, withdrawal, tolerance, and relapse. therapy or treatment for addiction, it practices focused attention and open monitoring. Here a person can clear their mind by starting on focusing on specific thing ie. their breathing, but then they can work into the awareness of their mental and begin to reflect back on the process or on their quality of
therapy or treatment for addiction, it practices focused attention and open monitoring. Here a person can clear their mind by starting on focusing on specific thing ie. their breathing, but then they can work into the awareness of their mental and begin to reflect back on the process or on their quality of 


 Why Do People Become Addicted to Drugs?
Why Do People Become Addicted to Drugs? Risk Factors for Relapse
Risk Factors for Relapse

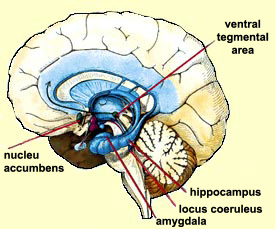 addicted have more than likely developed changes in their neural circuits that can give rise to new meanings and reward perceptions to drugs/shopping/technology/etc.
addicted have more than likely developed changes in their neural circuits that can give rise to new meanings and reward perceptions to drugs/shopping/technology/etc.  With the rise of technology, what is our world going to look like in 5,10, 20 years from now? What are we going to do to prevent this? Is anything actually needed to be prevented? It only seems like a matter of time before there are ways for diagnosing not only substance addictions but behavioral addictions too. Until then, we might just have to continue on with our days, checking our phones once they “ding” with a notification, briefly pausing, thinking, “Wait, do I have an addiction?”.
With the rise of technology, what is our world going to look like in 5,10, 20 years from now? What are we going to do to prevent this? Is anything actually needed to be prevented? It only seems like a matter of time before there are ways for diagnosing not only substance addictions but behavioral addictions too. Until then, we might just have to continue on with our days, checking our phones once they “ding” with a notification, briefly pausing, thinking, “Wait, do I have an addiction?”.
 Symptoms of food addiction are similar to drug addiction: losing control of what, when, and how much food is consumed and becoming dependent on food when experiencing stress or negative emotions. For people who try to break out of food addiction through diets and even medical procedures, relapsing into disordered eating habits is very likely.
Symptoms of food addiction are similar to drug addiction: losing control of what, when, and how much food is consumed and becoming dependent on food when experiencing stress or negative emotions. For people who try to break out of food addiction through diets and even medical procedures, relapsing into disordered eating habits is very likely.