It is painfully aware to us that as technology advances and society modernizes, obesity is becoming a severe epidemic. Why did obesity appear as we became more advanced? It is due to the increased access to a high fat diet. People have more access to low cost, high-fat foods and lower access to healthy, low-fat foods because healthier foods are more expensive. This has resulted in more and more people eating a high fat diet which will cause you to gain weight and fat which can lead to obesity. However, an interesting facet of this high fat diet and the path to obesity is how your brain changes and becomes imbalanced.
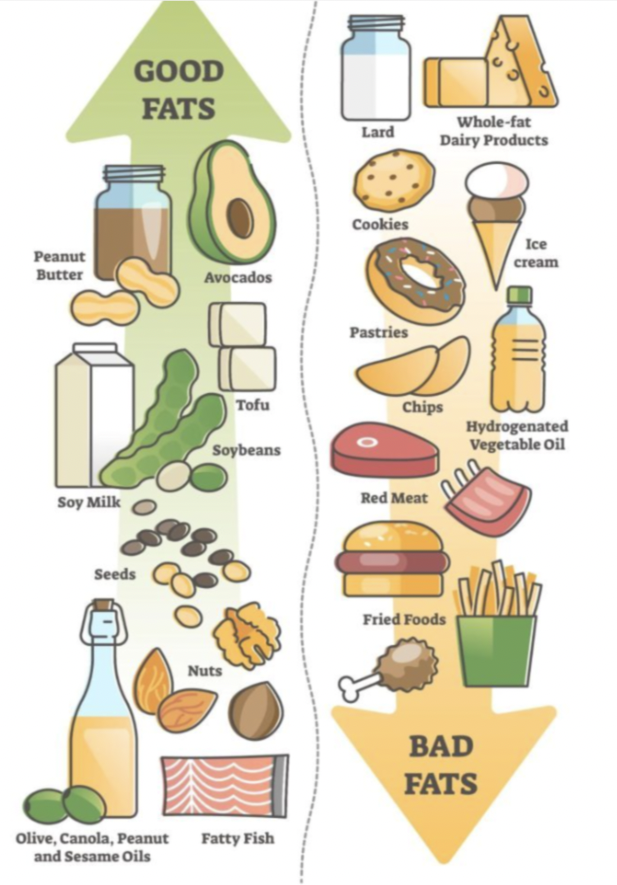
First, let us define a high fat diet. You may have heard of “good vs bad” or “healthy vs unhealthy” fats, the medical term is “unsaturated vs saturated” fats. Saturated and unsaturated fats have distinct structures and can perform different functions in the body. For example, saturated fats can stack up like a box of toothpicks and clog up arteries which harms blood flow. To learn more visit this link.
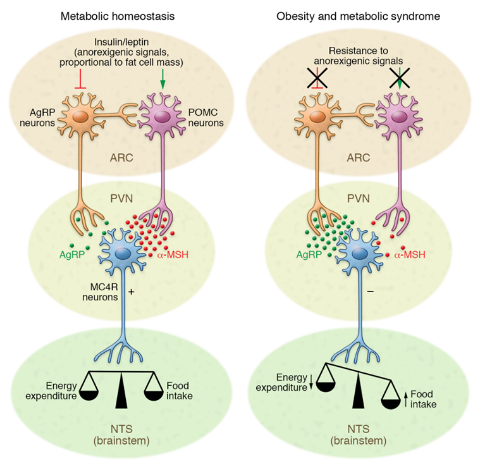
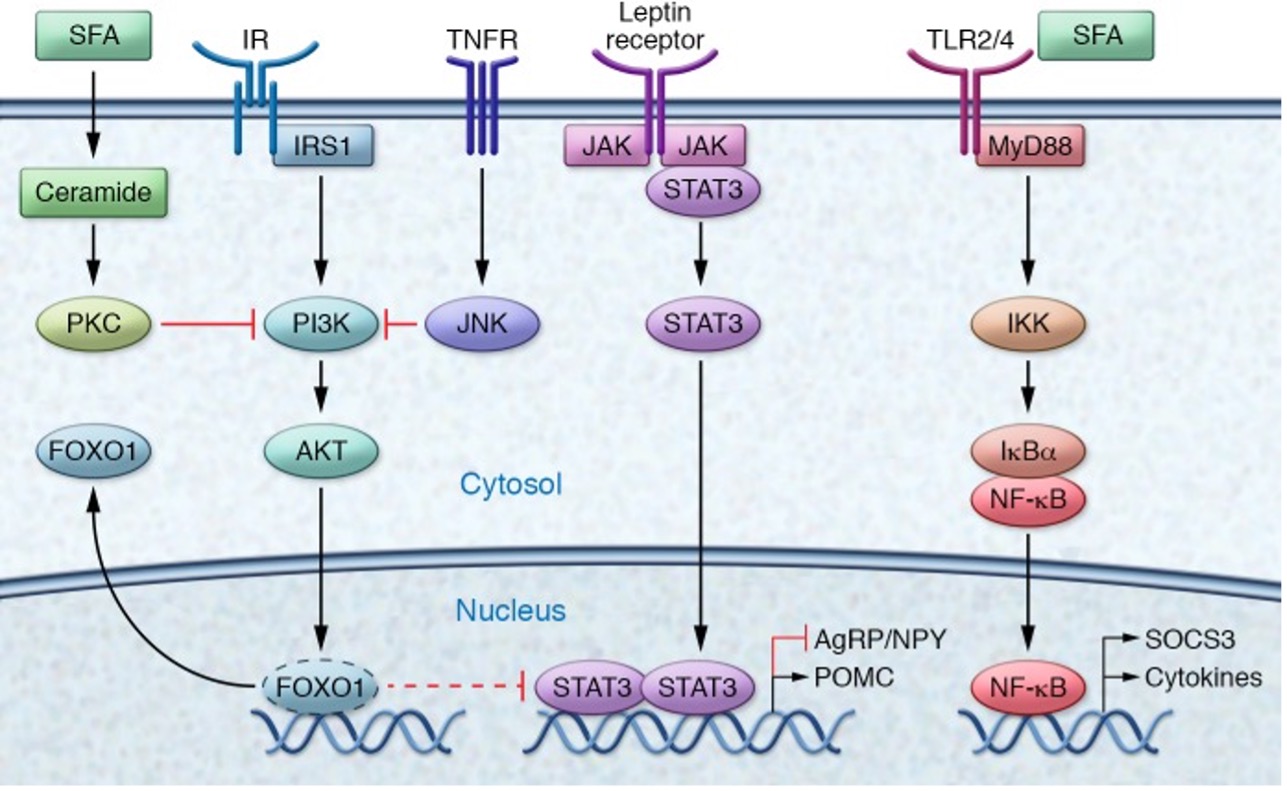
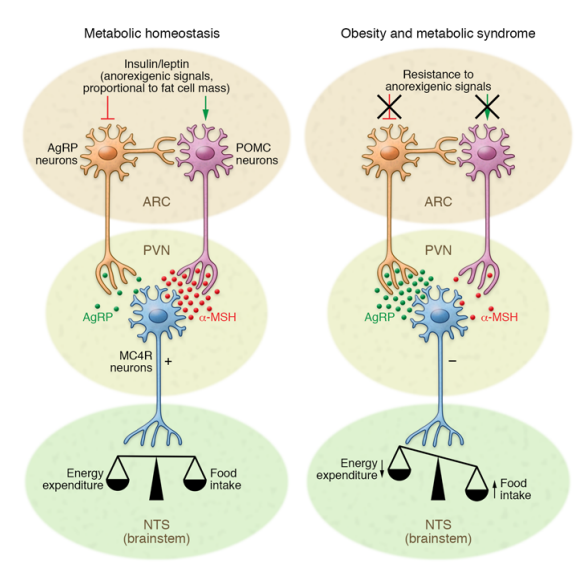
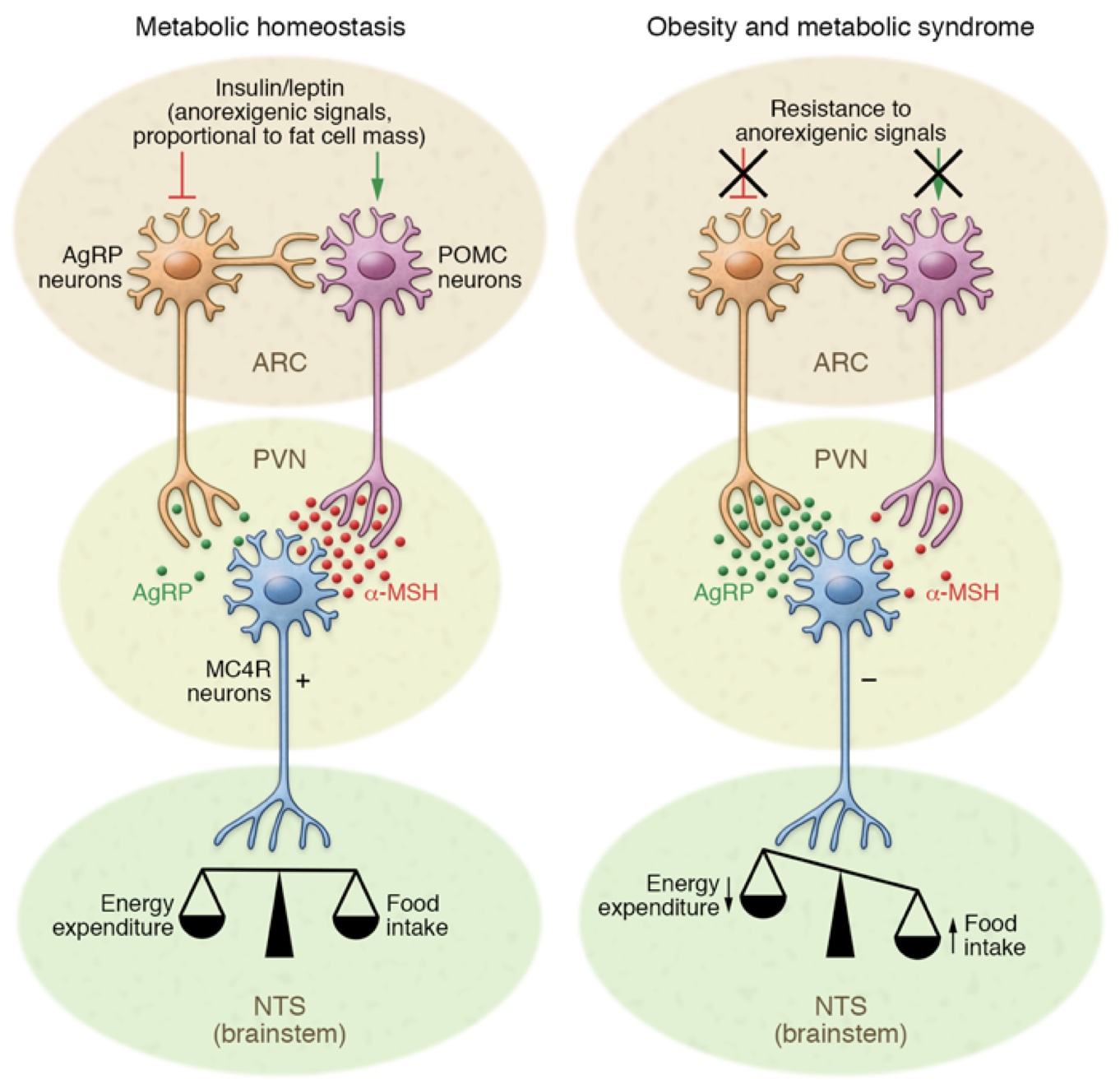
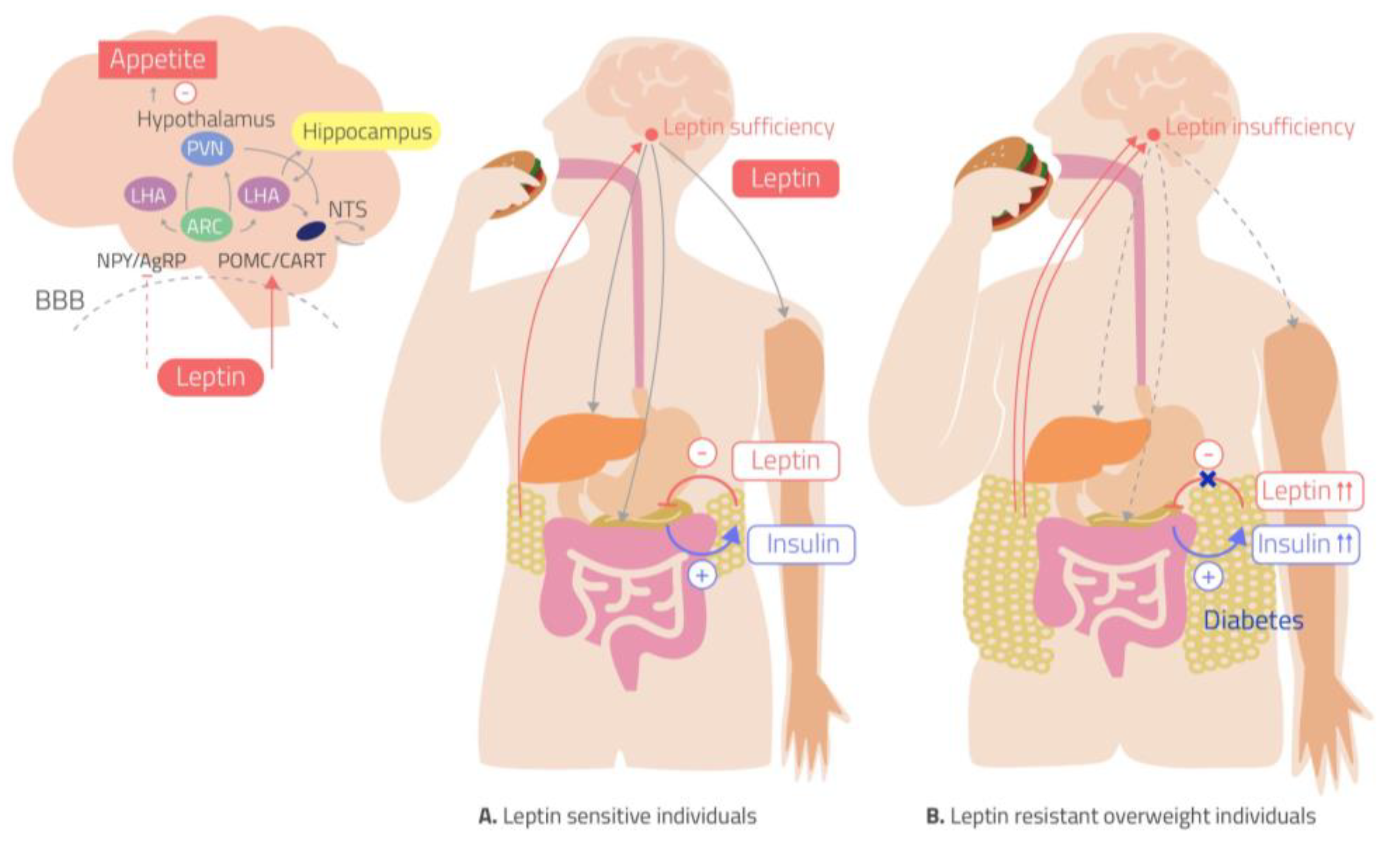
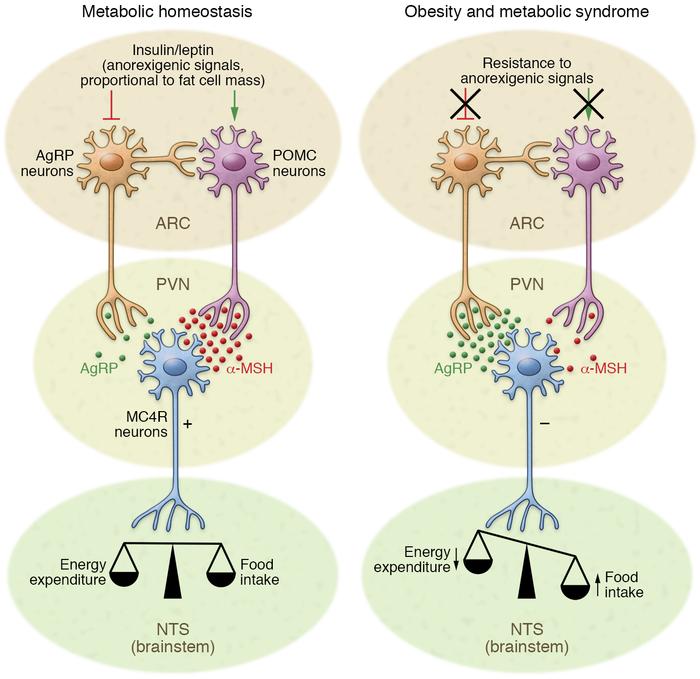
In your brain, there is one part of it that controls your hunger and thirst signals. This is the hypothalamus (Figure 1). The hypothalamus is what will get messages from the rest of your organs saying “I don’t have enough energy!” and send signals to your brain telling you to eat; Or the opposite can happen and your body says “I have all the energy I need.” and you will feel satisfied based off of the signals from the hypothalamus. What are these mysterious signals? The brain does not communicate through letters or text messages, the brain communicates chemically and the signals the hypothalamus uses are hormones. The two major hormones to discuss are insulin and leptin. They both end up telling the hypothalamus that no more energy is needed and the body can stop eating. However, in a high fat diet and obesity, these two hormones are not functioning as they should; let’s find out why.
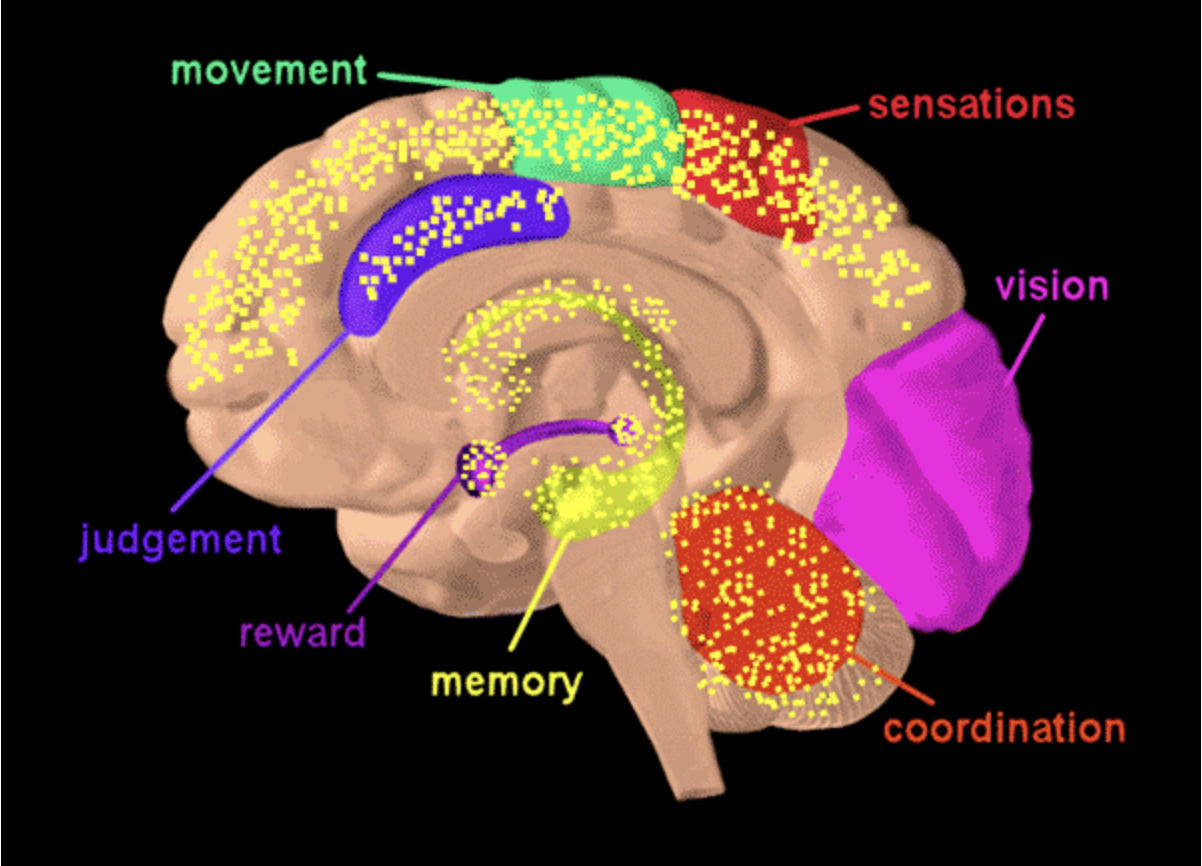
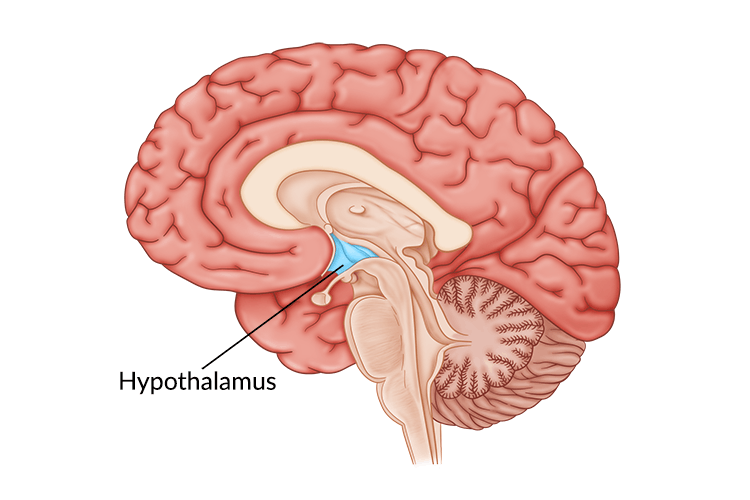 Figure 1: Diagram of the brain with the hypothalamus highlighted in blue. The hypothalamus is deep in the central part of the brain.
Figure 1: Diagram of the brain with the hypothalamus highlighted in blue. The hypothalamus is deep in the central part of the brain.
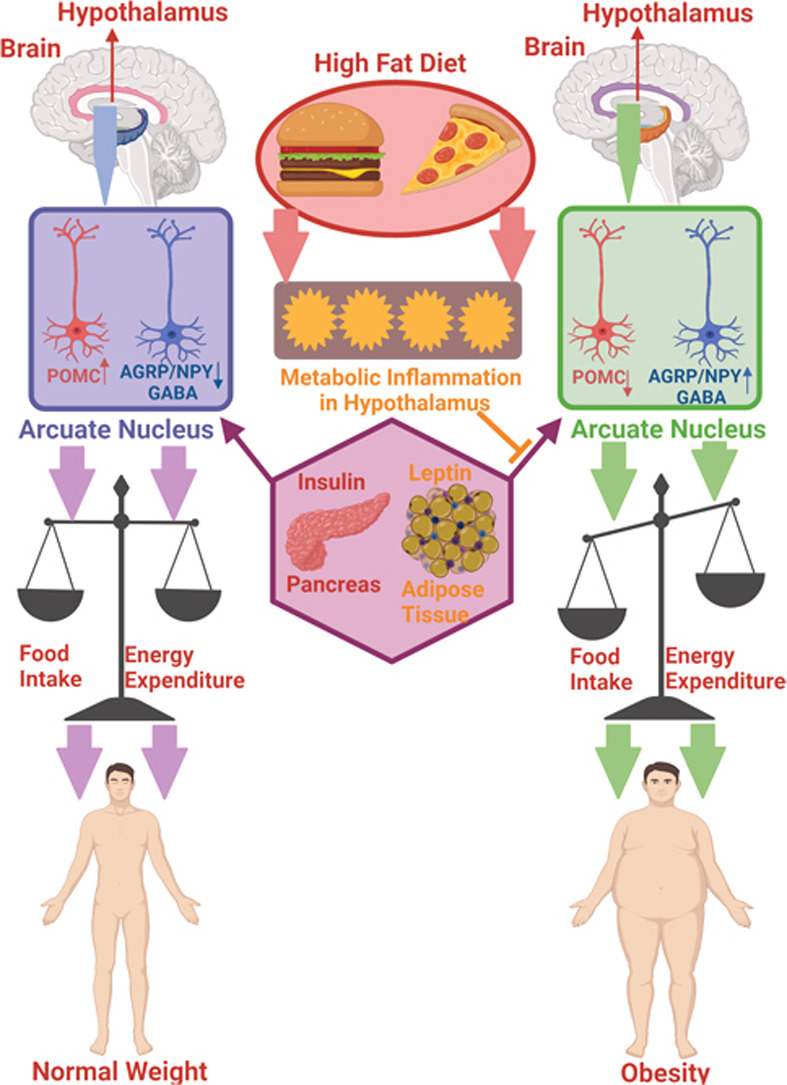
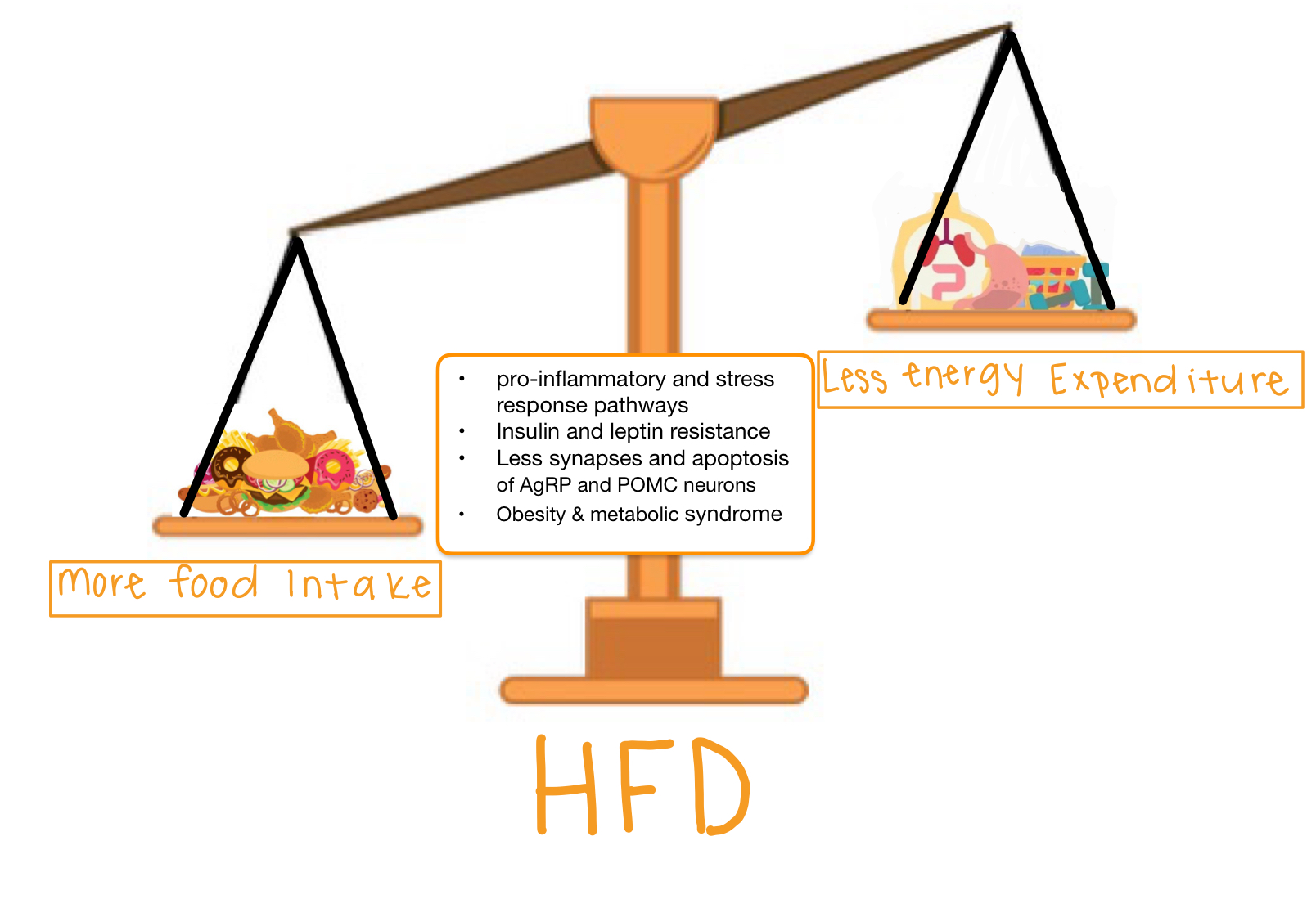
The saturated fatty acids from a high fat diet can actually cross the blood brain barrier which allows them to enter the brain and accumulate. Unsurprisingly, they accumulate mostly in the hypothalamus where they can block these hormones from reaching their receptors. Why is it bad if insulin and leptin can’t send their messages to the hypothalamus? If insulin and leptin are telling your body to stop eating, but they can’t get the message across, your brain will tell you to keep eating. You are feeling the pangs of hunger, even though your body has enough energy. This can result in overfeeding which leads to obesity. This can be called insensitivity to insulin which is a major part of diabetes; by giving the body more insulin, usually via injection, a bigger insulin signal is formed which means there is a higher chance the insulin signal can be received.
So know that a high fat diet is not only dangerous because of the weight gain and the accumulation of fat in the body, but it can also impact your brain function. Unfortunately, this impact will cause someone to keep overeating which can create a vicious cycle.
References:
Jais, A., & Brüning, J. C. (2017). Hypothalamic inflammation in obesity and metabolic disease. Journal of Clinical Investigation, 127(1), 24–32. https://doi.org/10.1172/JCI88878
Hypothalamus Damage: Causes, Symptoms, and Treatment. (2022, September 8). Flint Rehab. https://www.flintrehab.com/hypothalamus-brain-injury/
Hypothalamus: What It Is, Function, Conditions & Disorders. (n.d.). Cleveland Clinic. Retrieved April 22, 2024, from https://my.clevelandclinic.org/health/body/22566-hypothalamus
Learn the Key Differences Between Saturated and Unsaturated Fats. (n.d.). Verywell Health. Retrieved April 22, 2024, from https://www.verywellhealth.com/difference-between-saturated-fats-and-unsaturated-fats-697517
Temple, N. J. (2022). The Origins of the Obesity Epidemic in the USA–Lessons for Today. Nutrients, 14(20), 4253. https://doi.org/10.3390/nu14204253